Non-Alcoholic Fatty Liver Disease (NAFLD)
Non-Alcoholic Fatty Liver Disease (NAFLD)
Last Section Update: 04/2024
Contributor(s): Maureen Williams, ND; Carrie Decker, ND, MS; Shayna Sandhaus, PhD; Stephen Tapanes, PhD
Table of Contents
- Overview
- What Is Non-Alcoholic Fatty Liver Disease?
- Structure and Function of the Liver
- NAFLD Development & Progression
- NAFLD Risk Factors
- Nutrients
- Diet & Lifestyle Changes for NAFLD Prevention & Treatment
- Diagnosing NAFLD
- NAFLD Treatment
- Emerging Therapies
- Living with NAFLD
- Frequently Asked Questions About NAFLD
- Update History
- References
1 Overview
Summary & Quick Facts for NAFLD
- Non-alcoholic fatty liver disease (NAFLD) is the most common chronic liver disorder, estimated to affect about 35% of North Americans1; it affects closer to 80% of people who have metabolic syndrome.2
- An estimated 3–4% of the US adults have a more serious form of NAFLD, called non-alcoholic steatohepatitis or NASH.3
- NASH is associated with increased risks of fibrosis, cirrhosis, liver failure, and liver cancer.
- Diet and lifestyle changes to promote weight loss and improvements in sugar and fat metabolism are essential to the treatment of NAFLD.
- Many nutrients, such as vitamin E, milk thistle, berberine, curcumin, melatonin, probiotics, and omega-3 fatty acids, have been found to benefit those with NAFLD in clinical trials.
- One medication (resmetirom [Rezdiffra]) is specifically approved for NASH, and other medications and procedures used to treat conditions like obesity and type 2 diabetes, in conjunction with a healthy diet and lifestyle, may help combat NAFLD.
2 What Is Non-Alcoholic Fatty Liver Disease?
Non-alcoholic fatty liver disease (NAFLD), sometimes called fatty liver, is a common condition in which excess fat build-up occurs in the liver even if you drink little or no alcohol. NAFLD is typically caused by overeating, especially too much sugar and saturated fat.
In its early stages, NAFLD generally does not cause symptoms. In fact, many people—and their doctors—are unaware they have NAFLD. However, it can lead to liver damage over time in some people.
Fortunately, screening techniques such as blood levels of liver enzymes, ultrasound, and imaging techniques for assessing liver stiffness can help detect NAFLD, and the condition can often be reversed in its early stages with dietary and lifestyle changes.
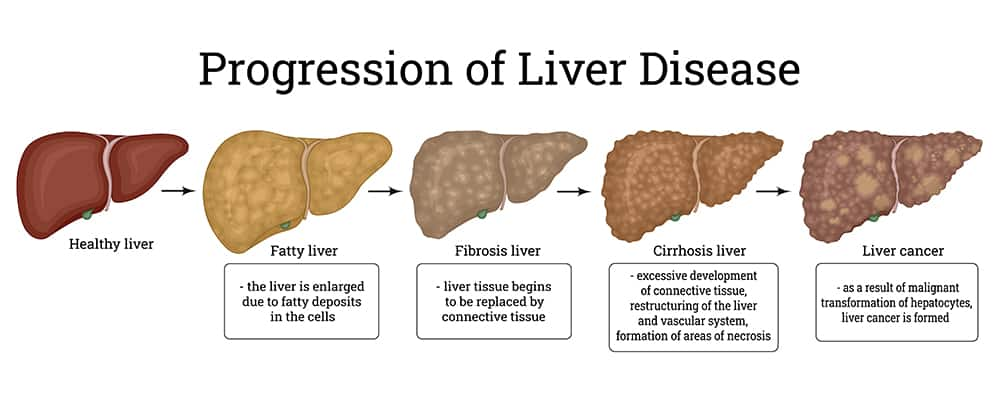
NAFLD is a chronic progressive condition with characteristics that define its early and late stages:
- The early stage, non-alcoholic fatty liver (NAFL or hepatic steatosis), in which excess fat is accumulating in the liver, and
- The advanced stage, non-alcoholic steatohepatitis (NASH), a more advanced form of the condition in which fat accumulation is accompanied by liver inflammation and liver cell damage.
The liver is remarkably resilient, able to repair and regenerate itself even after substantial cellular injury.4 If conditions that promote cellular injury are addressed, liver tissue affected by NAFLD can begin to heal, interrupting the progression of NAFLD to cirrhosis or liver cancer.5,6 Timely intervention with diet and lifestyle changes and nutritional support can slow or reverse fat accumulation and liver damage, even in some cases with evident inflammation and fibrosis (scarring).
3 Structure and Function of the Liver
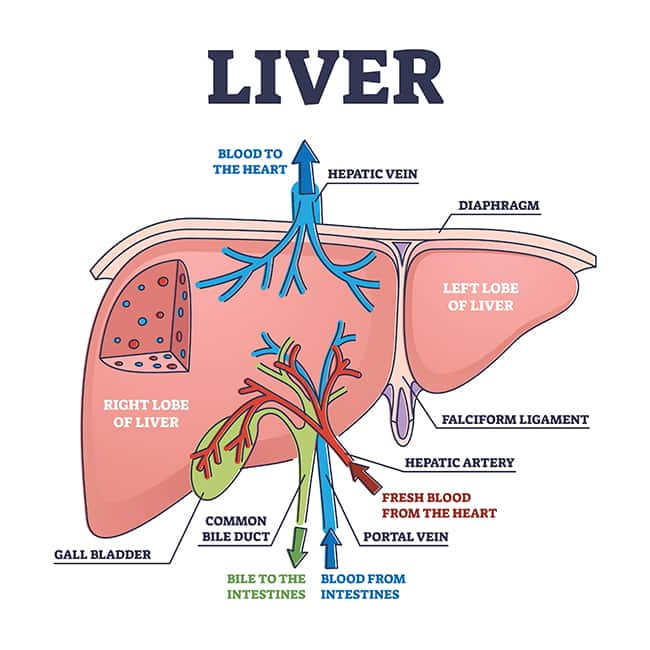
The liver sits behind the lower rib in the upper-right of the abdominal cavity. It is anatomically divided into two major lobes (right and left) and two smaller lobes (caudate and quadrate) that are supplied by branches of the hepatic artery, which brings oxygenated blood, and the portal vein, which brings nutrient-rich blood from the digestive tract. A network of bile ducts join and connect the liver to the gallbladder, directly below the liver, and the small intestine.7,8
The liver interacts with almost all body systems. It participates in digestion and elimination, detoxification, immune function, metabolism, nutrient storage, and more.7 For example, hepatocytes (the liver’s main cells) carry out functions such as7,8:
- Producing and secreting bile to aid in fat digestion
- Regulating storage and release of glucose and fats
- Producing and breaking down amino acids
- Producing blood proteins like albumin and coagulation factors
- Producing cholesterol
- Transforming and detoxifying steroid hormones and many drugs
Hepatocytes are organized into functional units called lobules, which are held together by a structural matrix of collagen and other proteins. Stellate cells (specialized cells that store fats and synthesize collagen and other structural proteins) are also present within the matrix surrounding the hepatocytes.7,8
4 NAFLD Development & Progression
Disordered fat metabolism is the fundamental mechanism underlying NAFLD. This dysfunctional fat metabolism is generally the result of diet-induced insulin resistance, which itself is usually the result of years of eating too much (and unhealthy) food.9
Normally, most dietary fatty acids are stored in adipose (fat) tissues in the form of triglycerides. Under healthy conditions, adipose triglycerides are broken down, releasing free fatty acids as needed for use in cellular energy production and to make lipid compounds such as those found in cell membranes. However, insulin resistance and associated metabolic dysfunction can lead to release of excess free fatty acids into the bloodstream, buildup of free fatty acids and triglycerides in the liver, and bloating of hepatocytes with too much fat.3,10
NAFLD is characterized by lipid accumulation in at least 5% of hepatocytes:
- When lipid accumulation occurs in the absence of other hepatocyte abnormalities, it is simply called non-alcoholic fatty liver (NAFL) or hepatic steatosis.
- Lipid accumulation accompanied by hepatocyte injury, inflammation, and fibrosis indicates a more advanced form of the condition called non-alcoholic steatohepatitis (NASH).3,11
- NASH with progressive fibrosis can eventually lead to cirrhosis and liver failure; it also increases the risk of primary liver cancer (hepatocellular carcinoma).10
Multiple factors likely contribute to the progression from NAFL to NASH and fibrosis. Some factors believed to lead to hepatocyte injury and inflammation include3,10,12:
- Worsening insulin resistance
- Imbalanced production of certain endogenous ceramides (while ceramides are important for skin integrity, in other tissues they can disrupt fat metabolism and their accumulation in the liver contributes to hepatocyte damage) (Note that supplemental, plant-derived ceramides, sometimes used to support skin health, differ from endogenous ceramides)
- Increased levels of oxidized lipids and oxidative stress in hepatocytes
- Gut microbiome disturbance and disrupted intestinal barrier function
- Mitochondrial dysfunction
- Inflammatory immune cell activation
Emerging preclinical research also shows degeneration of nerves in the liver occurs in the early stages of NAFLD and becomes more pronounced as the condition progresses. These nerves help regulate critical liver functions including glucose and lipid metabolism, bile production, blood flow, inflammatory signaling, and tissue regeneration, and their deterioration may both result from and contribute to advancing liver dysfunction.13
Progression of Fatty Liver
Chronic liver cell injury and inflammation can stimulate stellate cells to increase collagen production, leading to fibrosis (scarring) and eventually cirrhosis (severe and likely irreversible fibrosis).2 In addition, the conditions that contribute to the progression of NAFLD to NASH and fibrosis also increase the risk of hepatocellular carcinoma (primary liver cancer).10
Cirrhosis and liver failure. Inflammation and increased oxidative stress related to NASH accelerate liver cell damage and trigger fibrotic processes that can progress to cirrhosis and liver failure, a terminal condition treatable only with liver transplant.12 Nearly 68% of NASH patients will develop advanced fibrosis and 20% will develop cirrhosis.11,14 NAFLD patients who also have type 2 diabetes mellitus have the highest risk of cirrhosis and liver failure.12
Hepatocellular carcinoma. As liver cells undergo increasing rates of injury and death, production of new hepatocytes is up-regulated. This, combined with heightened inflammation and fibrosis, is believed to contribute to an increased risk of hepatocellular carcinoma.10 The likelihood of hepatocellular carcinoma over a 10-year period is estimated to be greater than 5% in patients with NASH, but is about 0.4% in those in the overall NAFLD-affected population.11 It is important to note that even NASH patients without cirrhosis may be at an elevated risk of hepatocellular carcinoma.10
5 NAFLD Risk Factors
Poor metabolic health contributes to most NAFLD cases. Health problems like obesity, insulin resistance, and belly fat accumulation are major contributors to the liver changes that occur in NAFLD. In fact, as research in recent years clarified the central role of disordered metabolism in NAFLD, experts proposed a new name for the condition: metabolic dysfunction-associated fatty liver disease (MAFLD).9
Obesity
Obesity is strongly associated with NAFLD. Weight loss, whether through diet or surgery, has been shown to improve NAFL and NASH in the large majority of patients.3 Excess fat tissue can become dysfunctional, leading to inflammation and insulin resistance, both of which contribute to liver fat accumulation.15
Insulin Resistance
Under healthy conditions, insulin signaling in fat tissue suppresses the breakdown of fat stores and release of free fatty acids. However, in insulin resistance (such as occurs in pre-diabetes and diabetes), lipid breakdown is unrestrained, blood levels of free fatty acids rise, and fat accumulation in the liver increases. Moreover, this rise in free fatty acid levels may contribute to disordered glucose production in the liver, worsening insulin resistance and increasing risks of type 2 diabetes and obesity.3
Visceral Adiposity (Belly Fat Accumulation)
Too much visceral adipose tissue is associated with inflammation. Visceral fat stores are also more prone to insulin resistance than subcutaneous fat.3 Visceral adiposity can occur in individuals without overweight or obesity, who represent almost 20% of those with NAFLD.16
Certain Medications
Some drugs can impair glucose and lipid metabolism and increase fat accumulation in the liver. Examples include corticosteroids, antidepressants, antipsychotics, and tamoxifen (Nolvadex, Soltamox) (a selective estrogen receptor modulator). In addition, methotrexate (MTX) (an immunosuppressant) and amiodarone (Pacerone) (an antiarrhythmic) can cause liver inflammation, injury, fibrosis, and cirrhosis.2,21 Some over-the-counter medications, like acetaminophen (Tylenol), can pose liver-health risks as well, particularly if used chronically and at high dosages.
Western Diet
A Western-style diet—specifically high in added sugars, red meat, saturated fat, and trans fats—increases the likelihood of metabolic disturbance and liver fat accumulation.20
Nutritional Derangement
Chronic overeating, severe malnutrition, and total parenteral nutrition are all associated with increased NAFLD risk.2
Alcohol Use
Even light-to-moderate alcohol consumption accelerates liver damage in those with NAFLD.20 Those with NAFLD should avoid alcohol consumption to minimize their chances of developing alcohol-related liver damage.
Smoking
Smoking has been associated with NAFLD in several studies. Moreover, passive or second-hand smoking has been associated with NAFLD as well.36
Gut Microbiome Imbalances
Imbalances in gut microbiome composition may result in problems with lipid and glucose metabolism, increased intestinal permeability, and liver inflammation and fibrosis.20
Non-modifiable factors that may contribute to NAFLD risk include:
- Age. The risk of NAFLD and its complications increases with age.20 Nevertheless, NAFLD affects people of all ages, and is the most common chronic liver disease in adolescents and children.52
- Gender. Estrogen appears to provide some protection against NAFLD; therefore, reproductive-aged women have a lower risk of NAFLD than similarly aged men, but their risk increases after menopause.20 Preclinical evidence suggests estradiol (the most active type of human estrogen) supports normal fat metabolism, suppresses fat production and accumulation in the liver, reduces inflammatory signaling in the liver, and inhibits liver fibrosis.53 Furthermore, observational and clinical studies indicate postmenopausal hormone replacement therapy is associated with lower liver enzyme levels, protection against metabolic dysfunction, and lower risk of NAFLD.54,55
- Family history. Having a parent or sibling with NAFLD is associated with increased risk.20
- Ethnicity. African Americans have a lower risk of NAFLD than Hispanics and non-Hispanic whites.20,56
- Genetics and epigenetics. Gene-environment interactions (epigenetics) can alter the way genes are expressed. Epigenetic changes due to diet, lifestyle, weight gain or loss, and other environmental factors can affect NAFLD risk in an individual and possibly their children. Certain gene variants associated with higher risk of NAFLD have also been identified.20 In addition, rare genetic disorders such as glycogen storage diseases, homocystinuria, and Wilson disease, as well as celiac disease (a genetic disease estimated to occur in about 1.3% of Americans) increase NAFLD risk.2
Health Conditions Associated with NAFLD
NAFLD affects metabolic function throughout the body and is linked to a wide range of chronic metabolic and inflammatory conditions. These include:
- Heart Disease
- NAFLD and cardiovascular disease share common risk factors, including obesity, visceral adiposity, insulin resistance, high blood pressure, high triglyceride and LDL-cholesterol levels, and low HDL-cholesterol levels.15
- Type 2 Diabetes
- People with type 2 diabetes have an increased risk of NAFLD, NASH, liver fibrosis, and cirrhosis.12
- Metabolic Syndrome
- NAFLD is marked by dysregulated metabolism of glucose and fats. As such, it is closely associated with metabolic syndrome, a co-occurring group of cardiometabolic risk factors (the presence of at least three risk criteria including high blood pressure, high triglyceride levels, low HDL-cholesterol levels, high fasting blood glucose, and abdominal obesity.10
- Polycystic Ovary Syndrome
- Polycystic ovary syndrome (PCOS) is a hormonal disorder affecting women. It is characterized by high androgen (male hormone) levels and abnormalities in ovarian structure and function. PCOS frequently includes metabolic features related to insulin resistance. Women with PCOS have greater risk of NAFLD.57
- Hypothyroidism
- NAFLD is more common in people with hypothyroidism, especially those with lower levels of free T4 (a thyroid hormone), older individuals, and those with overweight or obesity.58
- Depression, Anxiety, and Chronic Stress
- Metabolic syndrome, a condition closely correlated with NAFLD, is bi-directionally associated with mental health disorders, particularly depression and anxiety, as well as chronic stress.59 NAFLD may even be associated with depression and anxiety independently of other conditions.60
- Obstructive Sleep Apnea
- Obstructive sleep apnea (OSA) has been linked to NAFLD in multiple studies.61-64 The relationship between OSA and NAFLD may be bi-directional, with the occurrence of each increasing the risk of the other.62,65
- Periodontal Disease
- Periodontal disease, an inflammatory and infectious disease affecting the gums and bone that support the teeth, may contribute to NAFLD.66,67
- Sarcopenia
- Sarcopenia, loss of muscle mass, strength, and function that occurs with increasing age, involves some of the same metabolic conditions as NAFLD. NAFLD patients with sarcopenia, especially those who also have obesity, have a higher risk of progressing to NASH and developing other complications.68
- Chronic Kidney Disease
- Chronic kidney disease and NAFLD often occur together and have overlapping risk factors and metabolic mechanisms. Their relationship appears to be bi-directional, with each increasing the risk and severity of the other.69-71
6 Nutrients
Note: Insulin resistance is a fundamental driver of the aberrant fat metabolism that leads to lipid accumulation in the liver. Therefore, readers of this Protocol should also consult the Nutrients section of Life Extension’s Diabetes and Glucose Control Protocol to learn about nutrients that support healthy insulin and glucose metabolism. Similarly, because weight loss is a central pillar of NAFLD treatment, readers are encouraged to also consult Life Extension’s Weight Management Protocol.
Probiotics
Probiotics are live microorganisms that when consumed in adequate amounts promote health. They may benefit individuals with NAFLD by repairing intestinal barrier function, alleviating dysbiosis in the gut microbiome, reducing endotoxin-induced liver injury, and supporting healthy metabolism and immune function.51 Probiotics have demonstrated positive impacts in multiple clinical trials in patients with NAFLD or NASH. A meta-analysis of nine randomized controlled trials with a combined total of 352 subjects with NAFLD found probiotic therapy reduced liver enzyme and total cholesterol levels. Trials at least three months in duration showed that probiotics reduced body mass index (BMI) as well.72
A clinical trial randomized 52 individuals with NAFLD to either a placebo or “synbiotic” supplement twice daily for 28 weeks. The synbiotic comprised 200 million colony forming units (CFUs) of a combination of seven bacteria from the Lactobacillus, Bifidobacterium, and Streptococcus genera, as well as 125 mg of fructooligosaccharide (a prebiotic). Both the placebo and synbiotic groups were advised to eat a balanced diet and engage in regular physical activity. At the end of the trial, participants in the synbiotic group exhibited greater reductions in liver enzyme levels, inflammatory marker levels, and ultrasound-measured fibrosis than those who had taken the placebo.73 A similar trial in 50 normal-weight individuals with NAFLD found the same probiotic/prebiotic regimen resulted in greater reductions in liver fat and fibrosis and improvements in markers of inflammation and metabolism than placebo.80
A 12-week randomized controlled trial that included 68 participants with NAFLD and obesity compared the effects of a mixture of six probiotic bacteria (L. acidophilus, L. rhamnosus, L. paracasei, Pediococcus pentosaceus, B. lactis , and B. breve) with placebo. Probiotic treatment was found to reduce liver fat and fibrosis, and these effects appeared to be mediated by weight loss.74 A randomized controlled trial that included 50 patients with biopsy-diagnosed NASH found treatment with 100 million CFUs L. reuteri plus 4 grams of prebiotic fiber (guar gum and inulin) twice daily for three months reduced liver steatosis as assessed by magnetic resonance imaging, as well as body weight, BMI, and waist circumference, although it did not improve intestinal permeability or blood endotoxin levels.75 In another trial, 58 patients with NAFLD and type 2 diabetes received more than 1 trillion CFUs of a mixture of 14 probiotic bacterial species made up of Bifidobacterium, Lactobacillus, Lactococcus, Propionibacterium, and Acetobacter strains or placebo daily for eight weeks. Those receiving the probiotics had decreases in liver enzymes and some markers of inflammation, as well as fatty liver index scores.76
A 12-week trial in 111 participants with NAFLD found probiotic therapy, using a dosage of 5 billion CFUs of a combination of five Lactobacillus and Bifidobacterium strains twice daily, led to reductions in liver enzymes and triglyceride levels compared with placebo.77 In a trial in 30 NAFLD patients, 500 million CFUs of a combination of L. bulgaricus and S. thermophilus daily for three months reduced liver enzyme levels more than placebo.78
A supplement providing 1 billion spores per day of another probiotic strain, Bacillus coagulans, along with inulin, was found to reduce liver fat (assessed using ultrasound technology) as well as levels of markers of inflammation in a 12-week placebo-controlled trial that included 53 people with NAFLD.79
However, not all clinical trials have found positive effects. In a six-month trial in 39 patients with NAFLD, a multi-strain probiotic containing 30 billion CFUs of a combination of three Lactobacillus and three Bifidobacterium strains did not affect any NAFLD or metabolic parameters, though it did improve markers of intestinal permeability.81 Another randomized placebo-controlled trial in 35 NAFLD patients did not find a beneficial effect of a mixture of eight probiotic strains of Lactobacillus, Bifidobacterium, and Streptococcus species, at a dose of 1.8 trillion CFU twice daily, on markers of cardiovascular or liver health after 10 weeks.82 Future research is needed to identify optimal probiotic strains and doses for improving outcomes in NAFLD.
Vitamin E
Vitamin E helps lower oxidative stress by preventing lipid oxidation and has been widely studied for its possible benefits in NAFLD as well as NASH, the more severe form of the disease. A meta-analysis of eight randomized controlled trials in adults with NAFLD found vitamin E improved liver pathology and reduced liver enzyme levels better than placebo. In addition, vitamin E supplementation lowered LDL-cholesterol and fasting blood glucose, and improved levels of leptin, a metabolic hormone that plays a role in appetite and weight management.83 Another meta-analysis included five randomized controlled trials in adults with NAFLD lasting between three months and four years in which the treatment group received vitamin E at doses of 80–1,000 IU (53–670 mg d-alpha tocopherol equivalent) daily in conjunction with other therapies. The analysis found the inclusion of vitamin E in treatment improved liver enzyme levels and reduced signs of fatty liver, including on biopsy-based assessments.84 Vitamin E plus pioglitazone (Actos) (an anti-diabetes drug) in particular has been shown in multiple clinical trials to effectively treat NASH and reduce fibrosis.85 Other drugs that have shown promising effects when combined with vitamin E include pentoxifylline (Pentoxil, Trental)86 and spironolactone (Aldactone).87 Some emerging evidence suggests people carrying a certain variant of the haptoglobin (a protein produced by the liver) gene may be more likely than those with other variants to benefit from vitamin E therapy.88 If research continues to support this connection, genetic testing may help guide therapy in the future.
Tocotrienols, members of the vitamin E family along with the better-known tocopherols, have also been studied for their effects on NAFLD. In a randomized placebo-controlled trial in 71 people with NAFLD, 300 mg delta-tocotrienol twice daily for 24 weeks reduced insulin resistance and fatty liver index scores. In addition, those receiving delta-tocotrienol experienced improvements in liver enzyme levels, markers of inflammation and oxidative stress, and ultrasound assessments of liver steatosis.89 In another trial, 87 participants with NAFLD received either 200 mg mixed tocotrienols (including 61 mg alpha-tocopherol) twice daily or placebo for one year. Those given tocotrienols were more likely to have a normal liver ultrasound at the end of the trial.90
Milk Thistle
Milk thistle (Silybum marianum) has a long history of use as a liver protectant and detoxicant. Silymarin is a complex antioxidant extracted from milk thistle made up of six major flavonoid compounds and is a major active component of milk thistle.91 A 2021 meta-analysis included eight randomized placebo-controlled trials with a combined total of 622 participants. The trials lasted between 8 and 48 months and used dosages ranging from 140 mg to 2,100 mg of silymarin daily, with most trials using a daily dosage of 280 mg. The analysis found silymarin reduced liver enzyme levels better than placebo in individuals with NAFLD,92 which also was supported by a 2017 meta-analysis.93 In a randomized placebo-controlled trial that included 99 NASH patients, liver biopsies showed 22.4% of those treated with 700 mg silymarin three times daily for 48 weeks had a reduction in fibrosis versus only 6% who received placebo.94
Silymarin has also been studied in combination with other nutrients in individuals with NAFLD. One randomized controlled trial that included 78 participants with metabolic syndrome and NAFLD found 90 days of treatment with a supplement providing 250 mg silymarin plus 60 IU vitamin E daily, along with a diet and exercise program, reduced waist circumference, BMI, liver size, and scores on non-invasive measures of NAFLD compared with diet and exercise alone.95 A similar trial with 36 participants further noted diet and exercise plus the silymarin/vitamin E supplement reduced gamma-glutamyl transferase (GGT) (an enzyme, blood levels of which may be elevated with liver damage) levels and improved indices of NAFLD in the absence of weight loss, while diet and exercise alone did not.96
A combination of 750 mg silymarin, 200 IU vitamin E, and 1,000 mg L-carnitine per day for 18 weeks reduced blood glucose and insulin levels and improved insulin sensitivity scores more than placebo in a small trial with 25 participants. The participants had NAFLD, metabolic syndrome, and elevated liver enzyme levels.97 In a trial with 81 NAFLD-affected participants, diet and lifestyle modification along with a supplement providing 350 mg milk thistle extract (containing 280 mg silymarin), 120 mg vitamin C, 40 mg vitamin E, 20 mg coenzyme Q10, and 83 mcg selenium for 90 days improved liver enzyme and blood lipid levels more than diet and lifestyle alone. In addition, 51.3% of supplemented participants had large reductions in liver steatosis based on ultrasound imaging, while similar reductions were seen in only 15% of non-supplemented participants.98 A separate uncontrolled trial that followed-up over 1,700 people with NAFLD found similar benefits of a combination of vitamin C, CoQ10, selenium, and lipoic acid.99
Silibinin (or silybin), an active component of silymarin, has also received research attention as a potential therapy for NAFLD. A controlled trial that included 90 subjects with NAFLD found those treated with 303 mg silibinin along with 10 mcg (400 IU) vitamin D and 15 mg vitamin E, twice daily for six months, were more likely to improve with regard to metabolic markers, oxidative stress, endothelial function, and NAFLD progression compared with those who were untreated. The effect of the supplement was greater in participants who also had metabolic syndrome.100 In a study that included 270 people with NAFLD and chronic hepatitis B infection, 70 mg silibinin three times daily along with a diet and lifestyle program improved ultrasound-based evaluation of fatty liver more than diet and lifestyle alone after 24 weeks.101
Berberine
Berberine, an alkaloid chemical found in a number of medicinal plants, including Oregon grape (Mahonia aquifolium), barberry (Berberis vulgaris), goldenseal (Hydrastis canadensis), and gold thread (Coptis chinensis), has historically been used to treat infections and liver ailments.102 A range of health-promoting actions have more recently been attributed to berberine, including decreasing oxidative stress, modulating immune function, reducing inflammation, regulating carbohydrate and fat metabolism, improving mitochondrial function, and affecting epigenetic and microbiome factors.102,103 Preclinical evidence suggests berberine may be a promising therapeutic for NAFLD and NASH.103
A meta-analysis included findings from six controlled trials, with a total of 501 participants, in which berberine or berberine plus metformin was compared with lifestyle intervention or metformin or other anti-diabetes medications alone. The trials ranged from 12 to 18 weeks, and dosages of 300 or 500 mg berberine three times per day were used. The analysis found berberine reduced triglyceride levels more than other interventions and had positive effects on blood lipid, glucose, and liver enzyme levels, as well as insulin resistance and steatosis.104 One trial compared the effect of berberine therapy with lifestyle interventions to lifestyle interventions alone on NAFLD parameters as well as circulating lipid levels in 80 subjects with type 2 diabetes and NAFLD. After 16 weeks, the berberine-treated group had greater reductions in body weight, BMI, waist circumference, liver fat content, and cholesterol and triglyceride levels, and greater improvement in glucose tolerance. In addition, berberine therapy markedly reduced levels of ceramides, lipid compounds that can be produced in excess by metabolically dysfunctional fat tissue and accumulate in the liver where they trigger hepatocyte injury (though ceramides are safe and functionally critical in the skin).105 A combination supplement containing the salt of berberine and ursodeoxycholic acid (Actigall) (a bile acid used to treat liver and gallbladder ailments), at a dosage of 1,000 mg twice daily, was found to reduce liver fat content, liver enzyme levels, and body weight, and improve blood glucose control more than placebo after 18 weeks in a trial that included 100 subjects with type 2 diabetes, overweight or obesity, and NAFLD, with a high likelihood of NASH.106
Although berberine has been studied in human clinical trials and shown to have several metabolic benefits, concerns about long-term use of berberine have been raised on the basis of certain preclinical studies.107-109 Some evidence suggests that long-term berberine use, especially at high doses, may impair particular aspects of cellular metabolism in specific types of cells. The implications of this preclinical research are yet to be determined by long-term human clinical trials, therefore Life Extension currently recommends short-term use of berberine.
Curcumin
Curcumin is a flavonoid found in the culinary spice turmeric (Curcuma longa) that possesses well-established free radical-scavenging and anti-inflammatory properties. A growing body of evidence suggests curcumin can positively affect metabolic health and has a potential role in the treatment of metabolic disorders including type 2 diabetes, PCOS, cardiovascular disease, metabolic syndrome, and NAFLD.110
Recent meta-analyses of findings from randomized controlled trials indicate curcumin can reduce levels of liver enzymes, total and LDL-cholesterol, glucose, insulin, and triglycerides, and improve insulin resistance, waist circumference, and BMI in NAFLD patients.111-114 However, two placebo-controlled trials found 1,500 mg curcumin and other curcuminoids per day in addition to lifestyle changes for 12 weeks did not lead to statistically significant improvements in NAFLD or markers of inflammation or cardiovascular risk when compared with lifestyle changes alone.115,116
One randomized controlled trial examined the effect of whole turmeric powder on NAFLD. The trial included 64 people with NAFLD who received either 2 grams turmeric daily or placebo. After eight weeks, turmeric reduced liver enzymes more effectively than placebo but did not affect liver steatosis, as measured on ultrasound.117 Although curcumin has poor bioavailability when taken as unmodified turmeric powder, research suggests that in conditions related to gastrointestinal health (such as NAFLD) there may be gut-mediated mechanisms at play in the observed beneficial effects.118
Because of its poor bioavailability, various methods of preparation have been used to enhance curcumin absorption, such as adding piperine (a black pepper alkaloid), surrounding it with various types of nano-lipid envelopes, or complexing it with nano-phospholipids to create phytosomes.119 In a placebo-controlled study in 60 NAFLD patients, 500 mg curcumin with 5 mg piperine for 12 weeks decreased waist circumference, fasting blood glucose, LDL cholesterol, total cholesterol, and liver enzyme levels. Liver fibrosis and steatosis, however, were not changed with the treatment.298 On the other hand, a randomized controlled trial in 70 NAFLD patients found 500 mg curcumin and related curcuminoids plus 5 mg piperine per day for 12 weeks resulted in greater reduction in liver steatosis severity, assessed using ultrasound technology, than placebo.120 Similar trials also noted that 500 mg curcumin plus 5 mg piperine daily for two months reduced the severity of NAFLD more than placebo.121,122
Curcumin phytosomes in doses up to 1,500 mg daily have been shown to benefit patients with NAFLD in multiple clinical studies.299,300 A curcumin phytosome, at a dose of 250 mg (equivalent to 50 mg curcumin) daily for two months, was found to reduce liver fat and liver enzyme levels in a placebo-controlled trial in 80 NAFLD patients.123 In a placebo-controlled trial that included 80 individuals with overweight and impaired fasting glucose (prediabetes), a similar phospholipid-bound curcumin preparation, providing 200 mg curcumin, 600 mg phospholipids, and 8 mg piperine per day, was found to reduce liver enzyme levels and improve indices of NAFLD, as well as measures of glucose and lipid metabolism, after 56 days.124 In a randomized controlled trial, 84 participants with NAFLD and overweight or obesity received 40 mg of nano-encapsulated curcumin twice daily or placebo; after three months, those taking curcumin had decreased liver steatosis as assessed by ultrasound, as well as improvements in waist circumference and markers of inflammation and glucose and lipid metabolism.125
Melatonin
Melatonin, a hormone produced mainly by the pineal gland in the brain, is a critical regulator of the body’s circadian (sleep-wake) cycles. It also plays important roles in decreasing oxidative stress, inhibiting inflammatory signaling, modulating mitochondrial function, and normalizing cell proliferation and cell death.126,127 Based on its known actions, supplemental melatonin has been studied for its potential as a therapy for NAFLD.
Two meta-analyses indicated treatment with melatonin improved liver enzyme levels, particularly when used for four weeks or longer.128,129 In one randomized controlled trial in 45 participants, 6 mg melatonin daily one hour before bed for 12 weeks was more effective than placebo at decreasing NAFLD severity, body weight and waist circumference, and liver enzyme levels, and improving inflammatory and metabolic markers.130 In a trial in 42 patients with biopsy-diagnosed NASH, treatment with 5 mg melatonin twice daily for 12 weeks resulted in reductions in liver enzyme levels that were maintained during 12 more weeks of treatment but were mostly lost 12 weeks after stopping melatonin therapy.131,132 A 14-month randomized controlled trial that included 74 participants with biopsy-diagnosed NAFLD found melatonin, at 5 mg twice daily, reduced inflammatory cytokine levels, as well as GGT, triglyceride, and LDL-cholesterol levels; post-treatment biopsies performed in nine participants who volunteered for the procedure showed those with NASH who received treatment had resolution of liver injury and inflammation. Interestingly, participants in this trial who were treated with 500 mg tryptophan, the amino acid precursor of melatonin, twice daily experienced similar improvements as those who received melatonin.133
Omega-3 Fatty Acids and Specialized Pro-resolving Mediators
Omega-3 polyunsaturated fatty acids, especially eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA) from fatty fish and fish oil, are used in the body to synthesize compounds that resolve inflammatory immune activity and may improve metabolism in the liver, exerting protective effects in NAFLD.134-136
Findings from clinical trials evaluating the potential benefits of omega-3 fatty acids in individuals with NAFLD have yielded mixed results. One randomized controlled trial that included 56 subjects with type 2 diabetes and NAFLD found 1,000 mg omega-3 fatty acids (including 180 mg EPA and 120 mg DHA) twice daily for 12 weeks improved blood chemistries and body measurements related to NAFLD, leading to reductions in three indices used to assess NAFLD presence, but did not appear to reduce cardiovascular and metabolic risk factors.137,138 Findings from a small six-month randomized controlled trial that included 24 individuals with NAFLD suggest a fish oil supplement providing 1,509 mg DHA and 306 mg EPA may improve fibrosis, as assessed with an ultrasound-based technology.139 However, 1,250 mg of fish oil (providing 425 mg EPA and 325 mg DHA) twice daily did not improve liver enzyme levels, body composition, or other factors related to cardiovascular and metabolic health after 12 weeks in a randomized controlled trial in 104 NAFLD patients.140
Multiple meta-analyses have been performed to better understand the impact of omega-3 fatty acid supplementation on NAFLD. In general, these meta-analyses included findings from randomized controlled trials that lasted between two and 24 months and used doses of omega-3 fatty acids ranging from 250–6,000 mg daily. The analyses found NAFLD treatment with omega-3 fatty acids led to decreased liver steatosis and liver enzyme levels, as well as BMI, triglyceride and glucose levels, and insulin resistance, and improved other markers of metabolic status.141-143
Interestingly, the form of omega-3 used may influence the effects on factors related to NAFLD development and progression. A 2022 review of 38 academic papers concluded that preclinical research suggests omega-3 phospholipids have a more pronounced ability to combat fat accumulation in liver cells than omega-3s in triglyceride form. Alternative sources of marine omega-3 fatty acids, such as krill oil, generally provide omega-3s as phospholipid complexes. The authors of the review called for clinical trials to examine the effects of omega-3 phospholipids on NAFLD development and progression.144 Given that published evidence supports the use of traditional forms of omega-3, such as ethyl esters,136 in the context of NAFLD, a prudent choice may be to consume omega-3 phospholipids along with traditional forms.
Specialized pro-resolving mediators (SPMs) derived from omega-3 fatty acids and present in fish oil are also thought to play an important role in protecting liver health. SPMs such as resolvins, protectins, and maresins are well known to down-regulate and resolve inflammatory processes. Certain SPMs, including 17-hydroxydocosahexaenoic acid (17-HDHA), derived from DHA, and 18-hydroxyeicosapentaenoic acid (18-HEPE), derived from EPA, have also been found in preclinical research to reduce triglyceride accumulation in the liver, ameliorate fibrotic mechanisms, and improve metabolic parameters.134,135 However, clinical research to date in NAFLD has utilized omega-3 fatty acids and not SPMs directly, so the potential benefit of direct SPM therapy requires further study.
Green Tea
Green tea and its main active polyphenolic constituent, epigallocatechin gallate (EGCG), are well known for their anti-inflammatory and oxidative stress-reducing properties. Green tea also appears to modulate the gut microbiome and improve gut barrier function.145 Preclinical evidence indicates green tea extracts may alleviate metabolic dysfunction, reduce fibrosis, and inhibit the development of liver cancer, and may thereby help slow or reverse NAFLD progression.146
A randomized controlled trial that included 80 participants with ultrasound-diagnosed NAFLD and elevated liver enzyme levels found 500 mg green tea extract twice daily for 12 weeks reduced liver fat by 67.5% versus 25% with placebo. In addition, green tea extract led to improvements in body weight, BMI, and insulin resistance, and levels of liver enzymes, total cholesterol, LDL-cholesterol, HDL-cholesterol, triglycerides, and inflammatory markers.147 Another randomized controlled trial in 80 people with NAFLD found 500 mg green tea extract daily for 90 days reduced ALT and AST levels, while placebo did not.148
In a small randomized controlled trial that enrolled 17 participants diagnosed with NAFLD, drinking a catechin-enriched green tea providing 1,080 mg green tea catechin daily was found to have multiple positive effects: after 12 weeks, those who received the high-catechin tea had improvement in indirect measures of liver steatosis, lower body fat percentage, and decreased levels of ALT (a liver enzyme, high levels of which can indicate hepatocyte injury) and a marker of oxidative stress (urinary 8-isoprostane) compared with those who received a low-catechin tea or placebo.149
A 2018 meta-analysis of data from four clinical trials with a total of 234 participants found treatment with green tea or green tea catechins decreased liver enzyme, triglyceride, total cholesterol, and LDL-cholesterol levels in individuals with NAFLD.150
Alpha-Lipoic Acid
Alpha-lipoic acid is an antioxidant and antioxidant-recycling compound that has been found to decrease metabolic disease-related inflammation and reduce the risk of diabetes complications.151,152 Trials using lipoic acid as a monotherapy in participants with NAFLD have produced mixed results.153,154 However, lipoic acid combined with he bile acid ursodeoxycholic acid produced positive results in one trial. The trial included 120 participants with NAFLD who were advised to consume a low-calorie diet were assigned to one of four interventions for 12 months:
- 400 mg alpha-lipoic acid
- 300 mg ursodeoxycholic acid
- 400 mg alpha-lipoic acid plus 300 mg ursodeoxycholic acid
- placebo
At the end of the 12-month trial, participants taking both lipoic acid and ursodeoxycholic acid had greater decreases in liver enzyme levels, AST/ALT ratios, and liver fibrosis scores compared with those given either treatment alone or placebo. Benefits from the supplement combination were more pronounced in those who adhered to the low-calorie diet.155
N-acetylcysteine
N-acetylcysteine (NAC), a form of the amino acid cysteine, has well-known free radical-scavenging, anti-inflammatory, and anti-toxic effects in the liver. A number of preclinical studies have demonstrated NAC’s ability to limit fat accumulation and improve liver function by reducing oxidative stress and inflammation in animal models of NAFLD.156
In a clinical trial in 30 participants with NAFLD, those who received 600 mg NAC twice daily had greater reductions in levels of ALT than those given 1,000 mg vitamin C twice daily after three months. NAC therapy also led to decreased spleen size, which may indicate reduced fat infiltration.157 Another clinical trial in 53 patients with NASH found adding 1,200 mg of NAC per day to treatment with metformin for 48 weeks resulted in decreased ALT levels, liver fat, hepatocyte ballooning, and scores used to assess NAFLD activity compared with ursodeoxycholic acid or ursodeoxycholic acid plus NAC.158 One research team compared the effects of 600 mg NAC per day to no treatment in 35 NASH patients and reported their results in a letter. After four weeks, levels of three liver enzymes (ALT, AST, and GGT) decreased in those receiving NAC, while only ALT levels decreased with no treatment.159
Coenzyme Q10
Coenzyme Q10 (CoQ10) is a compound found throughout the body that participates in mitochondrial energy production, neutralizes free radicals, and restores antioxidants like vitamins C and E. CoQ10 has been most widely studied for its therapeutic effects in cardiovascular conditions, but it has also been shown to have important benefits in degenerative conditions and metabolic disorders.160 A meta-analysis of randomized controlled trials in 318 participants with metabolic syndrome, a condition closely linked to NAFLD, found CoQ10 supplementation increased levels of adiponectin, a metabolic hormone produced by fat tissue, and reduced levels of markers of inflammation and oxidative stress.161
Preclinical evidence suggests CoQ10 helps regulate lipid metabolism and inhibit accumulation of excess fat in the liver.160 CoQ10 may improve metabolism and slow the progression of NAFLD in part by ameliorating mitochondrial dysfunction, a possible contributing factor in NAFLD, and reducing liver oxidative stress and inflammation.162-164 In a randomized controlled trial that included 44 participants with NAFLD, 100 mg CoQ10 only had a significant effect on waist circumference, while body weight and blood markers of metabolic and liver health were not significantly different from those receiving placebo after four weeks, possibly due to the short duration of the trial.165 On the other hand, in a longer randomized controlled trial that enrolled 41 participants with NAFLD, 12 weeks of supplementation with CoQ10 (100 mg/day) decreased levels of liver enzymes and markers of inflammation, improved metabolic hormone levels, and reduced NAFLD (based on ultrasound assessments) more than placebo.166
Garlic
Garlic contains active organosulfur compounds thought to be responsible for its anti-inflammatory, cholesterol-lowering, and blood pressure-lowering effects.167 One research team published three reports based on a randomized controlled trial that enrolled 110 subjects with NAFLD and gave them either 400 mg of garlic powder twice daily for 15 weeks or placebo. Garlic was found to reduce blood pressure and levels of hs-CRP (high-sensitivity C-reactive protein) (a marker of inflammation),168 reduce ultrasound-measured liver steatosis, decrease liver enzyme levels, improve parameters related to lipid and glucose metabolism,169 and reduce body weight and body fat compared with placebo.170 A randomized controlled trial involving 90 participants found 1,600 mg garlic powder daily improved parameters of NAFLD and metabolic syndrome. Garlic treatment lowered blood pressure, waist circumference, GGT levels, triglyceride levels, insulin levels, insulin resistance, and appetite more than placebo after three months. In addition, garlic significantly decreased the fatty liver index, which is calculated using BMI, waist circumference, GGT, and triglycerides.171 The same research team reported that compared with placebo, 1,600 mg garlic daily for three months reduced liver fat (assessed by ultrasound) and improved liver enzyme levels and lipid profiles.172 It also decreased waist circumference and body fat, and improved markers of oxidative stress and glucose metabolism.173
Phosphatidylcholine
Choline, an essential nutrient found in food and the body mainly as the phospholipid phosphatidylcholine (PC), is necessary for fat metabolism and transport as well as cell structure, cell signaling, regulation of gene expression, and synthesis of the neurotransmitter acetylcholine.174 PC and other phospholipids are necessary for normal lipid metabolism in the liver, and human and animal studies have shown dietary choline deficiency promotes synthesis and accumulation of fat in the liver and increases NAFLD risk.174,175 Observational evidence suggests 1.8 grams PC daily, in conjunction with standard care, may be associated with reduced liver fat, decreased liver enzyme levels, and improved lipid profiles in people with NAFLD who had co-existing cardiometabolic disorders.176,177 One randomized controlled trial that included 29 individuals with NAFLD found treatment with 450 mg PC combined with 7 mg lycopene (for enhanced bioavailability of the PC) daily for two months was more effective than 450 mg PC alone at reducing liver enlargement on ultrasound exam.178
Carotenoids
Carotenoids are yellow and orange pigments found in many fruits and vegetables. In the body, carotenoids help protect lipids from oxidative damage and have been studied for their possible role in NAFLD prevention and treatment.179 A number of observational studies have noted lower intake and blood levels of carotenoids were associated with increased risk of NAFLD.180-183 In one longitudinal study that followed 2,687 middle-aged and elderly subjects with NAFLD for six years, higher blood levels of carotenoids (including β-carotene, α-carotene, β-cryptoxanthin, lycopene, and combined lutein plus zeaxanthin, as well as total carotenoids) at the beginning of the study were correlated with greater likelihood of NAFLD improvement without engaging in any specific intervention.184 Evidence from animal models of NAFLD also suggest carotenoids, such as β-carotene, lycopene, β-cryptoxanthin, lutein, zeaxanthin, astaxanthin, fucoxanthin, and crocetin, may have a beneficial effect on liver fat metabolism.181,185
In a randomized placebo-controlled trial that included 40 participants with NAFL or NASH, 3 mg of β-cryptoxanthin daily for 12 weeks lowered levels of the liver-related enzyme GGT and improved measures of oxidative stress and inflammation. The study authors also observed baseline β-cryptoxanthin levels were lower in NASH than NAFL patients.186
Ginger
Ginger is a culinary spice that has been proposed as a treatment for NAFLD due to its abilities to reduce inflammatory signaling, improve glucose and lipid metabolism, and decrease oxidative stress.187 In a randomized controlled trial that included 44 subjects with NAFLD, taking 1,000 mg ginger twice daily for 12 weeks lowered ALT and GGT levels, inflammatory cytokine levels, insulin resistance, and ultrasound-assessed liver fat, but did not affect AST levels or fibrosis relative to placebo.188 In another 12-week controlled trial of 46 participants with NAFLD, 500 mg of ginger powder three times daily resulted in greater reductions in levels of ALT, total and LDL-cholesterol, glucose, hs-CRP, and fetuin-A (a marker of metabolic disturbance) than placebo. It also improved insulin resistance. However, ginger had no effect on other parameters related to liver and metabolic health.189
French Oak Wood Extract
French oak wood extract has been studied and used for reducing fatigue and increasing energy. It has also been studied in the context of resolving liver damage.294
French oak wood extract may benefit liver cells because it contains polyphenols which can be converted in the body to bioactive metabolites called urolithins. In preclinical research, urolithin A has demonstrated favorable actions on mitophagy, which is the intracellular clearing of old, damaged mitochondria.294 Mitophagy is critical for maintaining cellular health and preventing disease.295 In addition, a pilot study in healthy volunteers indicated that 300 mg French oak wood extract for five days may also stimulate ribosomal biogenesis, which is essential for the creation of new proteins.296
In a controlled clinical interventional trial published in 2022, a total of 34 subjects with NAFLD were assigned to receive standard management with or without 200 mg French oak wood extract daily for three months. Standard management included controlling calories and salt and a total elimination of alcohol, caffeine, and any drugs. The parameters evaluated included liver enzymes, plasma glucose, a specialized type of liver ultrasound, BMI, fatigue and oxidative stress scores, and a laboratory index of liver fibrosis. After treatment completion, those in the oak wood group experienced larger decreases in BMI and fasting glucose than those in the control group. The oak wood intervention also resulted in significant improvement in the liver enzymes AST and ALT, and in the liver fibrosis index, compared to the standard management group. Improvement in these biomarkers is indicative of improved liver function. Results from the liver ultrasound revealed that those in the oak wood extract group, compared to the control group, had a significant decrease in the size, swollenness, and fibrosis of the liver. Those in the oak wood group also had significantly greater improvements in fatigue and oxidative stress scores compared with the control group.297
You Have A Fatty Liver. Now What? Part 2: Nutrients
7 Diet & Lifestyle Changes for NAFLD Prevention & Treatment
Dietary interventions and lifestyle changes, including adopting healthy eating habits, and getting regular physical activity, are the cornerstone to NAFLD prevention and treatment, while restricting alcohol use is also suggested to avoid alcohol-induced liver stress.190
Weight Loss
Weight loss is a key goal of lifestyle interventions in individuals with NAFLD who are overweight or obese. Numerous studies and clinical trials indicate loss of more than 5% of baseline body weight can reduce liver fat, and weight loss of 7% or more can reverse inflammation and fibrosis. Importantly, the degree of liver health improvement is generally proportional to the percentage of body weight lost in individuals with overweight/obesity.191
Consuming anywhere from 800 to 1,500 calories per day, with or without an exercise component, has generally been found to improve insulin resistance and parameters of liver health. The macronutrient composition of an anti-NAFLD diet may be less important than its calorie content, but there is more evidence of benefit from low-carbohydrate than low-fat diets.191,192 It is important to consult a healthcare provider with nutritional expertise before embarking on a very low-calorie diet. Generally, a gastroenterologist will treat individuals with liver disease.
Intermittent fasting is a dieting strategy that has been shown to induce weight loss and improve health status in those with metabolic diseases, including NAFLD. Intermittent fasting protocols typically include daily fasting for 12 hours or more (time-restricted eating), or intensive calorie restriction two days per week or on alternate days. Fasting is thought to help restore normal circadian cycles of metabolism and may have benefits beyond weight loss.192 A large meta-analysis concluded an intermittent fasting protocol based on 3–5 days per week of intensive calorie restriction was the most beneficial with regards to weight loss and metabolic improvement compared to regular diet or continuous calorie restriction.193
Healthy Eating Habits
A meta-analysis of findings from 18 observational studies with a total of 24,867 participants found Western dietary patterns, characterized by large amounts of processed food, red meat, high-fat dairy products, and refined grains, were associated with a higher risk of NAFLD. On the other hand, heart-healthy, high-fiber dietary interventions characterized by large amounts of fruits, vegetables, whole grains, and healthy fats like those found in fish and olive oil, such as the Mediterranean diet, were associated with a lower risk of NAFLD.194 Adherence to DASH (Dietary Approaches to Stop Hypertension) guidelines, which emphasize fruit, vegetables, whole grains, and low-fat dairy products, and limit saturated fat, cholesterol, and refined grains, has been found to improve metabolic health and is associated with a lower prevalence of NAFLD.196 Eating a fiber-rich plant-based diet has also been associated with a lower risk of NAFLD.197,198 Higher intakes of nuts and legumes specifically have been associated with lower likelihood of NAFLD.199,200 Nevertheless, some evidence suggests animal foods can be part of a therapeutic diet for recovering metabolic health. Early clinical trials indicate a Paleolithic diet, which emphasizes vegetables, fruit, nuts, eggs, fish, and lean meats, may help reverse obesity, type 2 diabetes, and NAFLD.201,202
The Mediterranean diet in particular has been shown in multiple trials to reduce waist circumference (a measure of abdominal obesity) and blood pressure, increase glucose tolerance and HDL-cholesterol levels, and decrease the risk of metabolic syndrome and death. Even in the absence of weight loss, a Mediterranean-style diet can improve insulin resistance, a key factor in NAFLD.192 In a randomized controlled trial, 294 obese participants with abnormal lipid levels, including 182 with NAFLD, were randomly assigned to a healthy diet, Mediterranean diet, or Mediterranean diet that included extra polyphenols (plant antioxidants). After 18 months, those in the Mediterranean diet groups lost 2‒3 times more liver fat than those in the healthy diet group.203
Sugar and Calories
Multiple studies have implicated high-sugar and high-calorie diets with the development of NAFLD.190,204,205 A clinical trial in 16 overweight subjects found adding 1,000 calories from sugary candy and soft drinks to the diet every day for three weeks led to a 2% increase in body weight and a dramatic 27% increase in liver fat accumulation. Conversely, six months of calorie restriction while emphasizing vegetables and whole grains resulted in a 4% weight loss and 25% loss of liver fat.206 Other clinical trials in adolescents and adults suggest limiting fructose intake, especially by cutting out soft drinks and foods with added sugars, can rapidly improve signs of NAFLD and markers of metabolic health.207-210
Fats
Saturated fat intake has been correlated with risk of NAFLD in numerous observational and clinical studies.224 In a clinical trial, 61 participants with overweight or obesity were randomly assigned to increase their daily caloric intake by adding muffins that provided an average of 40 grams per day of either palm oil (high in saturated fat) or sunflower oil (high in polyunsaturated fat) for eight weeks. Following the eight-week trial, the participants switched to a standardized very low-calorie (800 calories per day) diet using macronutrient-balanced meal replacement supplements for four weeks. The two diets resulted in similar weight gain. However, the saturated fat-enhanced diet led to increased liver fat accumulation and blood levels of LDL-cholesterol, ceramides, and liver enzymes, while the polyunsaturated fat-enhanced diet reduced liver fat stores, decreased LDL-cholesterol and ceramide levels, and had no effect on liver enzyme levels. All of the negative effects of the saturated fat-enriched diet were reversed during the four-week very low-calorie diet.225
Another trial in 38 overweight subjects compared the effects of adding 1,000 calories per day from saturated fat sources (such as coconut oil, butter, and cheese), mono- and polyunsaturated fat sources (such as olive oil, pesto, and pecans, as well as some butter), or simple sugars (such as from orange juice, sugar-sweetened beverages, and candy). After three weeks, those receiving extra saturated fat calories had the greatest increase in liver fat accumulation (55%), followed by those receiving simple sugar calories (33%), and those receiving unsaturated fat calories (15%). In addition, the added-saturated fat diet worsened markers of insulin resistance and raised blood ceramide levels.226 Even in a 10-week trial in which 67 abdominally obese subjects ate diets that were unchanged in calories and macronutrient balance, those given a higher proportion of polyunsaturated fat (from vegetable oil) for 10 weeks had reduced liver fat stores, and improved levels of insulin, cholesterol, and triglycerides compared with those given a higher proportion of saturated fat (from butter).227
Protein
The effects of protein on NAFLD are less well studied than those of carbohydrate and fat. A clinical trial in 37 subjects with NAFLD and type 2 diabetes found a high-protein diet, whether based on animal (meat and dairy) or plant (legume) protein, led to improvements in metabolic status and liver fat and inflammation after six weeks. The beneficial effects of the high-protein diets were unrelated to changes in body weight.228
Coffee
Although not all studies agree, coffee consumption appears to be correlated with lower risk of fibrosis in NAFLD patients.229-232 Observational data shows those with NAFLD who drank more than three cups of coffee per day had a lower risk of fibrosis than those who drank less than two cups per day. The evidence further suggests habitual coffee drinking by individuals with NAFLD may lower their risks of cirrhosis and hepatocellular cancer.233 In one large observational study that followed nearly 500,000 participants for about 11 years, drinking up to 3–4 cups per day of any type of coffee, including decaffeinated, instant, or ground coffee, was associated with lower risks of all types of chronic liver disease, including NAFLD, as well as cirrhosis and hepatocellular cancer.234
Eating Behaviors
Eating behaviors, such as nighttime eating, eating while rushed, and eating for comfort or to relieve stress, may have more harmful effects on metabolism than eating patterns that are synchronized with normal circadian cycles.192,235 A habit of eating before bedtime has been associated with NAFLD risk in observational research.236 Even metabolically healthy individuals have been shown to develop altered carbohydrate metabolism after eating a late dinner.237 Such evidence has contributed to the common recommendation to stop eating 3‒4 hours before bedtime. Other research suggests being a fast eater is correlated with higher risk of NAFLD, although one study determined this effect was likely due to increased weight gain in fast eaters.238,239
Exercise
Exercise is a key component of most weight loss programs. Current guidelines for NAFLD recommend moderate-intensity aerobic exercise for a total of 150 to 300 minutes per week, plus strength and endurance training 2‒3 times per week, as well as breaking up prolonged sedentary time with a few minutes of physical activity (eg, walking).240,241 Even more modest exercise habits can have beneficial effects on the risk of onset and progression of NAFLD. A study that followed 233,676 adults for five years found exercising five or more times per week for at least 10 minutes was associated with a reduced risk of developing NAFLD, as well as increased chance of fatty liver reversal in those with NAFLD at the beginning of the study.242 Another observational study that followed 5,860 individuals for 2.5 years also found becoming or remaining physically active was associated with lower odds of developing NAFLD and greater odds of NAFLD improvement compared with being physically inactive.243
Interventions that do not include dietary change or weight loss have demonstrated exercise alone can improve metabolic health in NAFLD patients. A systematic review and meta-analysis of 10 randomized controlled trials that included a total of 316 participants with NAFLD found exercise (aerobic, strength training, or a combination of both) without diet change or weight loss reduced liver steatosis and levels of liver enzymes, LDL-cholesterol, and triglycerides.244
It has been hypothesized that the benefit of exercise, independent of weight loss, may be related to increased muscle strength and function, decreased oxidative stress and inflammation, reduced insulin resistance, improved mitochondrial function, and possibly changes in gut microbiome composition.240,244 Exercise may also affect epigenetic mechanisms related to lipid metabolism in the liver.240 Although there is more evidence in support of the benefits of aerobic exercise, strength training is likely to have complementary positive effects.241
You Have a Fatty Liver. Now What?
8 Diagnosing NAFLD
Some people with NAFLD experience discomfort or pain in the upper right abdomen, fatigue, and other non-specific symptoms. However, most individuals with NAFLD have no symptoms. Therefore, awareness and screening are important for those at risk.2 Screening begins with a thorough medical and family history to identify the presence of risk factors and associated conditions. Blood tests and liver function tests are used to assess metabolic status and liver health, the results of which can be incorporated into non-invasive index models and scoring tools used to indicate the likelihood of NAFLD, NASH, and fibrosis. Imaging studies may be ordered to visualize fat accumulation and assess inflammation and fibrosis. If advanced fibrosis or cirrhosis is suspected, liver biopsy may be performed to confirm the diagnosis.245
Blood Tests
Common screening blood tests include248:
- Liver enzymes: AST (aspartate aminotransferase), ALT (alanine aminotransferase), and GGT (gamma-glutamyl transferase)249
- Lipids: Triglycerides, total cholesterol, high-density lipoprotein-cholesterol (HDL-C), and low-density lipoprotein-cholesterol (LDL-C)248
- Glucose metabolism markers: Glucose, insulin, and hemoglobin A1C (HbA1C)248
Screening Tools for NAFLD, NASH, and Fibrosis
A number of index models and scoring tools have been shown to be reasonably correlated with presence of NAFLD, although they have poor ability to stage severity or distinguish NAFL from NASH.248,250 Other index and scoring tools are useful for predicting the presence of fibrosis.248 Examples of widely used screening tools are251:
- Fatty Liver Index (FLI). FLI is calculated using BMI, waist circumference, triglycerides, and GGT.
- Hepatic Steatosis Index (HSI). HSI is determined by BMI, AST/ALT ratio, and type 2 diabetes status.
- NAFLD Liver Fat Score (LFS). The NAFLD LFS uses type 2 diabetes and metabolic syndrome status as well as AST/ALT ratio and insulin level to predict the presence of NAFLD.
- NAFLD Fibrosis Score (NFS). The NFS is based on age, BMI, high blood glucose level, platelet count, albumin level, and AST/ALT ratio.
- Fibrosis-4 Index (FIB-4). FIB-4 is a simple screening tool that uses age, AST, ALT, and platelet count to screen for advanced fibrosis.
Imaging
Ultrasound is commonly used in diagnosing fatty liver. Ultrasound can help distinguish the severity of steatosis based on the percentage of the liver affected, and is able to detect moderate-to-severe NAFLD with a high degree of accuracy; however, ultrasound is less sensitive in mild cases where fewer than 20% of hepatocytes are fat-laden, and its accuracy is reduced in individuals with obesity.248,250 A specialized ultrasound-based technology called transient elastography (Fibroscan), which assesses liver stiffness, can detect advanced fibrosis and cirrhosis with good to excellent accuracy and is the most widely used technique for this purpose.248 Its use in combination with the FIB-4 index has been demonstrated to be an effective, as well as cost-effective, approach to detecting and staging fibrosis and cirrhosis in NAFLD patients.252
Magnetic resonance (MR)-based diagnostic techniques are also available for evaluating liver status. These techniques have a high degree of accuracy for detecting all grades of fat accumulation, even in individuals with obesity.248,250 They may be particularly helpful in detecting and staging fibrosis, as well as identifying NASH. Despite the promising performance of MR-based diagnostic technologies, their usefulness is limited by high cost, complexity of use, and other factors.250
Biopsy
Biopsy is considered the gold standard for diagnosing and grading NAFLD, NASH, and fibrosis. However, because it carries a risk of rare but potentially life-threatening complications, is subject to sample variability (ie, samples may come from liver regions that are more or less affected), and is expensive, it is generally reserved for patients for whom medication or surgery is being considered and those whose condition is uncertain.2,250
9 NAFLD Treatment
There is one FDA-approved medical treatment for NASH (ie, resmetirom [Rezdiffra]).302 Additionally, people with NAFLD and obesity or type 2 diabetes may benefit from medical interventions, particularly if they are unable to achieve meaningful weight loss through dietary interventions and lifestyle therapies. These interventions include antiobesity and antidiabetes medications, endoscopic bariatric procedures, and bariatric surgery. Importantly, medical and surgical therapies are always recommended in conjunction with diet and lifestyle therapies, which remain the cornerstone of NAFLD treatment.253
Resmetirom for NASH
Resmetirom (Rezdiffra) is an oral thyroid hormone receptor-beta agonist. It was approved by the Food and Drug Administration in March 2024 and marks the first medication approved to treat NASH.302 A randomized, controlled, phase 2 trial enrolling patients with biopsy confirmed NASH (liver fibrosis stages 1–3) was conducted across 18 sites in the United States and published results in 2019. A total of 384 participants were screened, with 84 randomly assigned to receive 80 mg of resmetirom and 41 to receive placebo once daily for 36 weeks. Treatment with resmetirom resulted in a significant reduction in hepatic fat compared with placebo. Resmetirom significantly reduced hepatic fat by 32.9% and 37.3% at 12 weeks and 36 weeks, respectively, compared with 10.4% and 8.5% in the placebo group.282 A subsequent open label study was conducted in 31 patients from the original study with persistently mild to markedly elevated liver enzymes. Participants were given 80 or 100 mg of resmetirom daily. There was a significant reduction from baseline in the ratio of N‐terminal type III collagen propeptide to metalloproteinase‐degraded collagen III (PRO-C3/C3M), a marker of net fibrosis formation. Liver stiffness, LDL cholesterol, apolipoprotein B, and triglycerides were also significantly reduced from baseline.284
Resmetirom was tested in a phase 3 controlled study enrolling 966 participants randomized to receive 80 or 100 mg resmetirom or placebo for 52 weeks. The two primary endpoints in the study were NASH resolution and an improvement in fibrosis by at least one stage with no worsening of the NAFLD activity score. NASH resolution was achieved in 25.9% and 29.9% of patients in the 80 and 100 mg groups, respectively, compared with only 9.7% in the placebo group. Fibrosis improvement by at least one stage was achieved in 24.2% and 25.9% of patients in the 80 and 100 mg groups, respectively, compared with 14.2% in the placebo group. These represented significant improvements. There were also significant reductions in LDL cholesterol of 13.6% and 16.3% in the 80 and 100 mg resmetirom groups, respectively, compared with a 0.1% increase in the placebo group.303 These trials, along with a separate phase 3 trial evaluating safety over 52 weeks, found that resmetirom was safe and well tolerated in adults. In the separate phase 3 study assessing safety, liver enzymes were shown to be reduced and no signs or symptoms relating to hyperthyroidism, hypothyroidism, or central thyroid axis changes were observed with resmetirom, suggesting it does not affect thyroid hormone receptor-alpha.304
Antiobesity and Antidiabetes Medications
Drug therapies targeting obesity and type 2 diabetes, the conditions most closely associated with NAFLD, appear to be helpful in reducing NAFLD.253
Orlistat. Orlistat (Alli or Xenical), an oral drug that inhibits dietary fat digestion and absorption, is used to treat obesity. Several clinical trials have reported treatment with orlistat lasting 24 weeks or longer can induce weight loss of 5–10%, improve AST and ALT levels, and reduce biopsy-assessed liver fat content and fibrosis.253 Orlistat use can cause fat malabsorption and diarrhea.
Glucagon-like peptide-1 receptor agonists (GLP-1 RAs). GLP-1 is a hormone produced in the intestinal wall that decreases glucagon release and increases insulin release by the pancreas. It also slows stomach emptying, which can reduce food intake.253 GLP-1 RAs, administered as subcutaneous injections, are used to treat type 2 diabetes. A systematic review and meta-analysis of six randomized controlled trials in a combined total of 406 participants with NAFLD found treatment with GLP-1 analogs (eg, exenatide [Byetta or Bydureon] and liraglutide [Saxenda]) led to reductions in liver fat content and BMI, and increased levels of adiponectin (a hormone secreted by adipose tissue that helps regulate fat and glucose metabolism).254 Another meta-analysis that included data from 371 NAFLD patients participating in five randomized controlled trials found liraglutide significantly reduced BMI, especially in those with co-occurring type 2 diabetes, but its lowering effects on liver fat and ALT levels were not statistically significant.255
A randomized controlled trial in 320 NASH patients found 59% of those who received 72 weeks of treatment with the GLP-1 analog semaglutide (Ozempic or Wegovy) and 17% of those receiving placebo experienced resolution of biopsy-confirmed NASH, although changes in fibrosis were not significantly different between groups.256 An emerging drug called cotadutide, which works by activating both GLP-1 and glucagon receptors, reduced non-biopsy markers of NAFLD and fibrosis better than liraglutide in a 54-week randomized placebo-controlled trial in 834 adults with overweight or obesity and type 2 diabetes.257
GLP-1 analogs can cause digestive side effects such as nausea, vomiting, diarrhea or constipation, and abdominal pain.253
Pioglitazone. Pioglitazone (Actos) works by activating a nuclear receptor (peroxisome proliferator-activated receptor [PPAR]) involved in regulating cell metabolism. Pioglitazone was found to improve NAFLD and NASH recovery (but not fibrosis) in a meta-analysis of four randomized controlled trials in patients with prediabetes or type 2 diabetes and NAFLD that used biopsies to confirm liver fat content and inflammation status258; however, its usefulness is limited by its potential adverse side effects, which include water retention; weight gain; edema; heart failure; bone fracture; and chest, epigastric, back, and joint pain.258,259
Empagliflozin. Empagliflozin (Jardiance) is an antidiabetes drug that increases glucose excretion by the kidneys. Multiple placebo-controlled trials have found empagliflozin can reduce liver fat content and liver enzyme levels in individuals with NAFLD and type 2 diabetes.260-262
Endoscopic Bariatric Procedures
Endoscopic bariatric procedures are characterized by the use of a flexible endoscopic tube to install a device that reduces stomach capacity and thereby mechanically limits food intake. One such device is an intragastric balloon, which is inflated after insertion to reduce stomach volume. Clinical trials indicate intragastric balloons can improve steatosis, steatohepatitis, and fibrosis. They can also cause adverse side effects including abdominal pain, nausea, GERD, and other less common complications.253 Endoscopic sleeve gastroplasty is performed by endoscopically inserting a suturing device that is then used to section off part of the stomach. Non-invasive measures of fibrosis have also been found to improve following endoscopic sleeve gastroplasty.267,268 Although rare, serious side effects such as pain, nausea requiring hospitalization, bleeding, leaking, pulmonary embolism and pneumoperitoneum (air trapped in the membrane surrounding the abdominal cavity) from sleeve gastroplasty have been reported.253
Bariatric Surgery
Bariatric surgery is an option available to individuals with a BMI of 40 or higher, or a BMI of 35 or higher plus an obesity-related complication. Examples of commonly used bariatric surgeries are sleeve gastrectomy (sometimes referred to as “stomach stapling”) and Roux-en-Y gastric bypass. Bariatric surgery has been found to improve glucose and lipid metabolism independently of weight loss, and multiple studies indicate bariatric surgery may lead to resolution of NAFLD and NASH and reverse early-stage fibrosis.253,269 One observational study that followed 64 participants with biopsy-confirmed NASH for five years after bariatric surgery found that 84% had resolution of NASH, 70.2% had decreased fibrosis, and 56% had disappearance of fibrosis.270 Another study followed 1,158 subjects with obesity and biopsy-confirmed NASH and fibrosis and found the risk of a major adverse liver event was about 2.3% over 10 years in those who were treated with bariatric surgery and 9.6% in those who were not. In addition, the risk of major cardiovascular events was also lower in the surgically treated group.271
The potential benefits of bariatric surgery in patients with obesity and NAFLD need to be weighed against their potential harms. Although these surgeries are generally safe, they can cause devastating, and sometimes fatal, complications. These include chronic malnourishment, bowel obstruction, gallstones, hernia, and ulcers, as well as acute liver impairment and long-term worsening of NAFLD.253,272
10 Emerging Therapies
In light of NAFLD’s increasing prevalence, researchers are exploring a number of innovative strategies for reversing steatosis, reducing liver inflammation, and repairing liver injury.273 For example, the blood pressure-lowering drug telmisartan (Micardis), an angiotensin II receptor blocker (ARB), has been investigated for its potential benefits in NAFLD treatment. In addition to causing blood vessel constriction, increased activity of the enzyme angiotensin II can trigger increased oxidative stress, inflammation, and insulin resistance, and appears to be a major contributor to disordered lipid metabolism in the liver.274 Early clinical trials have reported telmisartan improved NAFLD and fibrosis scores, as well as evidence of steatosis and inflammation on liver biopsy, in NASH patients.275,276 Evidence suggests telmisartan’s positive effects on liver health may be due in part to its ability to stimulate peroxisome proliferator-activated receptors (PPARs).277
Some other drug strategies under development are outlined in Table 1.
| Table 1: Emerging Drugs for NAFLD Treatment273 | |||
|---|---|---|---|
| Category | Examples | Mechanism of Action | Early Evidence |
| Farnesoid X receptor agonists |
Ursodeoxycholic acid Obeticholic acid (Ocaliva) Tropifexor Cilofexor Nidufexor |
Improves regulation of lipid and glucose metabolism in the liver and increases liver repair |
Ursodeoxycholic acid: lowered ALT levels, but no effect on other biochemical markers of liver function278 Obeticholic acid: improved fibrosis, but no effect on NASH279 |
| Lipogenesis inhibitors |
Aramchol Firsocostat |
Inhibits activity of enzymes needed for fat synthesis in the liver |
Aramchol: Reduced liver fat content in NAFLD patients but not NASH patients280 Firsocostat: Reduced liver fat content in NASH patients281 |
| Liver anti-inflammatory agents | Cenicriviroc | Blocks specific inflammatory cytokine receptors in the liver | Reduced fibrosis, but no change in NASH, in patients with NASH and fibrosis; stronger effect in those with more advanced fibrosis285 |
| Fibroblast growth factor -19 (FGF19) analogs | Aldafermin | Mimics a gut hormone that regulates bile acid and carbohydrate metabolism | Reduced liver fat and improved fibrosis in NASH patients with moderate-to-severe fibrosis286 |
| Phosphodiesterase inhibitors | Pentoxifylline | Antioxidant and anti-inflammatory | Reduced liver fat, inflammation, and fibrosis in one trial in NASH patients,287 but only non-significant improvement in NASH patients in another trial288 |
| Cannabinoid receptor-1 antagonists | Rimonabant (Zimulti) Cannabidiol (CBD) Tetrahydrocannabivarin (THCV) | Reduces appetite and promotes energy expenditure, inducing weight loss; decreases liver inflammation and oxidative stress through uncertain mechanisms | Observed to improve insulin resistance and lipid profiles in obesity patients with NAFLD289 and reduce ALT levels and insulin resistance in women with PCOS290,291 |
11 Living with NAFLD
A diagnosis of NAFLD can be a wake-up call for people with unhealthy habits and may be just what is needed to inspire healthy, liver-friendly lifestyle changes. But long-term change is difficult, especially, as in NAFLD, when the results are not readily apparent. It is important to keep in mind that healthy changes in diet, exercise, and other daily routines have invisible benefits to your long-term health, even when they do not result in substantial hoped-for changes like weight loss.
Getting support through individual or group counseling can help build motivation and strengthen adherence to lifestyle programs aimed at reversing NAFLD. Researchers have found a hybrid in-person and web-based intervention can be a convenient and helpful alternative for providing structured support to NAFLD patients implementing lifestyle interventions.292 Another study showed weekly text messages providing educational information about healthy lifestyle was more effective than standard diet and exercise counseling in people with NAFLD trying to lose weight.293
In addition to frequent individualized support, periodic lab testing to monitor indirect measures of liver health, like liver enzyme levels, triglyceride and cholesterol levels, and blood markers of glucose control, as well as waist circumference and possibly liver imaging, may help you stay on track.
12 Frequently Asked Questions About NAFLD
How do I know if I have NAFLD?
Most people with NAFLD have no symptoms, but if you have other metabolic health problems like overweight or obesity, type 2 diabetes or prediabetes, excess belly fat, and high triglyceride and cholesterol levels, there is a good chance you have NAFLD. Your doctor may use body measurements and blood tests to determine your likelihood of having NAFLD. If there is a need to know for sure, your doctor will order a liver ultrasound, possibly followed by a liver biopsy.
What is the most common cause of NAFLD?
The most common cause of fatty liver is eating a Western-style diet, high in calories, saturated fat, trans fats, red meat, and added sugars. Drinking large amounts of sugary soft drinks is also linked to a high risk of developing NAFLD.
What problems can NAFLD cause?
Non-alcoholic fatty liver by itself often has no direct consequences, but in some cases, fatty liver progresses to an inflammatory liver condition (steatohepatitis) that compromises liver function and increases the risks of fibrosis, cirrhosis, and liver cancer.
What can I do to prevent NAFLD?
Adopt a healthy diet and lifestyle and maintain a healthy weight. Avoiding alcohol, which also can lead to liver disease, is also recommended.
How long does it take to heal NAFLD?
Liver health responds quickly to diet and lifestyle interventions. Most studies in people with NAFLD show positive diet and lifestyle changes can begin to reverse liver fat accumulation within 4–12 weeks.
2024
- Apr: Added section on resmetirom to NAFLD Treatment
2023
- Jul: Added sidebar on updated terminology to Overview
- Feb: Added section on French oak wood extract to Nutrients
2022
- Jun: Comprehensive update & review
2020
- Apr: Added section on probiotics and prebiotics to Nutrients
2010
- Oct: Comprehensive update & review
Disclaimer and Safety Information
This information (and any accompanying material) is not intended to replace the attention or advice of a physician or other qualified health care professional. Anyone who wishes to embark on any dietary, drug, exercise, or other lifestyle change intended to prevent or treat a specific disease or condition should first consult with and seek clearance from a physician or other qualified health care professional. Pregnant women in particular should seek the advice of a physician before using any protocol listed on this website. The protocols described on this website are for adults only, unless otherwise specified. Product labels may contain important safety information and the most recent product information provided by the product manufacturers should be carefully reviewed prior to use to verify the dose, administration, and contraindications. National, state, and local laws may vary regarding the use and application of many of the therapies discussed. The reader assumes the risk of any injuries. The authors and publishers, their affiliates and assigns are not liable for any injury and/or damage to persons arising from this protocol and expressly disclaim responsibility for any adverse effects resulting from the use of the information contained herein.
The protocols raise many issues that are subject to change as new data emerge. None of our suggested protocol regimens can guarantee health benefits. Life Extension has not performed independent verification of the data contained in the referenced materials, and expressly disclaims responsibility for any error in the literature.
- Le MH, Yeo YH, Li X, et al. 2019 Global NAFLD Prevalence: A Systematic Review and Meta-analysis. Clinical gastroenterology and hepatology : the official clinical practice journal of the American Gastroenterological Association . Dec 7 2021;doi:10.1016/j.cgh.2021.12.002. https://www.ncbi.nlm.nih.gov/pubmed/34890795
- Antunes C, Azadfard M, Hoilat GJ, Gupta M. Fatty Liver. StatPearls . StatPearls Publishing Copyright © 2022, StatPearls Publishing LLC.; 2022.
- Bence KK, Birnbaum MJ. Metabolic drivers of non-alcoholic fatty liver disease. Molecular metabolism. Aug 2021;50:101143. doi:10.1016/j.molmet.2020.101143. https://www.ncbi.nlm.nih.gov/pubmed/33346069
- Hadjittofi C, Feretis M, Martin J, Harper S, Huguet E. Liver regeneration biology: Implications for liver tumour therapies. World journal of clinical oncology. Dec 24 2021;12(12):1101-1156. doi:10.5306/wjco.v12.i12.1101. https://www.ncbi.nlm.nih.gov/pubmed/35070734
- Schuster S, Cabrera D, Arrese M, Feldstein AE. Triggering and resolution of inflammation in NASH. Nat Rev Gastroenterol Hepatol. Jun 2018;15(6):349-364. doi:10.1038/s41575-018-0009-6. https://www.ncbi.nlm.nih.gov/pubmed/29740166
- Albhaisi S, Noureddin M. Current and Potential Therapies Targeting Inflammation in NASH. Frontiers in endocrinology. 2021;12:767314. doi:10.3389/fendo.2021.767314. https://www.ncbi.nlm.nih.gov/pubmed/34925237
- Kalra A, Yetiskul E, Wehrle CJ, Tuma F. Physiology, Liver. StatPearls. StatPearls Publishing Copyright © 2022, StatPearls Publishing LLC.; 2022.
- Ozougwu J. Physiology of the liver. 01/01 2017;4:13-24.
- Powell EE, Wong VW, Rinella M. Non-alcoholic fatty liver disease. Lancet. Jun 5 2021;397(10290):2212-2224. doi:10.1016/S0140-6736(20)32511-3. https://www.ncbi.nlm.nih.gov/pubmed/33894145
- Chakravarthy MV, Neuschwander-Tetri BA. The metabolic basis of nonalcoholic steatohepatitis. Endocrinol Diabetes Metab. Oct 2020;3(4):e00112. doi:10.1002/edm2.112. https://www.ncbi.nlm.nih.gov/pubmed/33102794
- Younossi ZM, Koenig AB, Abdelatif D, Fazel Y, Henry L, Wymer M. Global epidemiology of nonalcoholic fatty liver disease-Meta-analytic assessment of prevalence, incidence, and outcomes. Hepatology (Baltimore, Md) . Jul 2016;64(1):73-84. doi:10.1002/hep.28431. https://www.ncbi.nlm.nih.gov/pubmed/26707365
- Ramai D, Facciorusso A, Vigandt E, et al. Progressive Liver Fibrosis in Non-Alcoholic Fatty Liver Disease. Cells. Dec 2 2021;10(12)doi:10.3390/cells10123401. https://www.ncbi.nlm.nih.gov/pubmed/34943908
- Adori C, Daraio T, Kuiper R, et al. Disorganization and degeneration of liver sympathetic innervations in nonalcoholic fatty liver disease revealed by 3D imaging. Science advances. Jul 2021;7(30)doi:10.1126/sciadv.abg5733. https://www.ncbi.nlm.nih.gov/pubmed/34290096
- Sheka AC, Adeyi O, Thompson J, Hameed B, Crawford PA, Ikramuddin S. Nonalcoholic Steatohepatitis: A Review. JAMA. Mar 24 2020;323(12):1175-1183. doi:10.1001/jama.2020.2298. https://www.ncbi.nlm.nih.gov/pubmed/32207804
- Gutierrez-Cuevas J, Santos A, Armendariz-Borunda J. Pathophysiological Molecular Mechanisms of Obesity: A Link between MAFLD and NASH with Cardiovascular Diseases. International journal of molecular sciences. Oct 27 2021;22(21)doi:10.3390/ijms222111629. https://www.ncbi.nlm.nih.gov/pubmed/34769060
- Ye Q, Zou B, Yeo YH, et al. Global prevalence, incidence, and outcomes of non-obese or lean non-alcoholic fatty liver disease: a systematic review and meta-analysis. Lancet Gastroenterol Hepatol. Aug 2020;5(8):739-752. doi:10.1016/S2468-1253(20)30077-7. https://www.ncbi.nlm.nih.gov/pubmed/32413340
- Sookoian S, Pirola CJ. Systematic review with meta-analysis: the significance of histological disease severity in lean patients with nonalcoholic fatty liver disease. Alimentary pharmacology & therapeutics. Jan 2018;47(1):16-25. doi:10.1111/apt.14401. https://www.ncbi.nlm.nih.gov/pubmed/29083036
- Kuchay MS, Martinez-Montoro JI, Choudhary NS, Fernandez-Garcia JC, Ramos-Molina B. Non-Alcoholic Fatty Liver Disease in Lean and Non-Obese Individuals: Current and Future Challenges. Biomedicines. Sep 28 2021;9(10)doi:10.3390/biomedicines9101346. https://www.ncbi.nlm.nih.gov/pubmed/34680463
- Lu FB, Zheng KI, Rios RS, Targher G, Byrne CD, Zheng MH. Global epidemiology of lean non-alcoholic fatty liver disease: A systematic review and meta-analysis. Journal of gastroenterology and hepatology. Dec 2020;35(12):2041-2050. doi:10.1111/jgh.15156. https://www.ncbi.nlm.nih.gov/pubmed/32573017
- Pal P, Palui R, Ray S. Heterogeneity of non-alcoholic fatty liver disease: Implications for clinical practice and research activity. World J Hepatol. Nov 27 2021;13(11):1584-1610. doi:10.4254/wjh.v13.i11.1584. https://www.ncbi.nlm.nih.gov/pubmed/34904031
- NIDDKD. LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet]. Bethesda (MD): National Institute of Diabetes and Digestive and Kidney Diseases; 2012-. Nonalcoholic Fatty Liver. Available at https://www.ncbi.nlm.nih.gov/books/NBK547860/. Updated 05/04/2019. Accessed 02/21/2022. 2019;
- Himoto T, Masaki T. Current Trends of Essential Trace Elements in Patients with Chronic Liver Diseases. Nutrients. Jul 14 2020;12(7)doi:10.3390/nu12072084. https://www.ncbi.nlm.nih.gov/pubmed/32674425
- Harber I, Zedan D, McClintock S, Varani J, Aslam M. Liver Proteomic Profile in Mice on a High-Fat Diet: Modulation with Anti-Tumor Intervention. Experimental Biology, poster presentation. 2022;
- Lee CY, Lee CL. Comparison of the Improvement Effect of Deep Ocean Water with Different Mineral Composition on the High Fat Diet-Induced Blood Lipid and Nonalcoholic Fatty Liver Disease in a Mouse Model. Nutrients. May 20 2021;13(5)doi:10.3390/nu13051732. https://www.ncbi.nlm.nih.gov/pubmed/34065270
- Kumar SA, Magnusson M, Ward LC, Paul NA, Brown L. Seaweed supplements normalise metabolic, cardiovascular and liver responses in high-carbohydrate, high-fat fed rats. Mar Drugs. Feb 2 2015;13(2):788-805. doi:10.3390/md13020788. https://www.ncbi.nlm.nih.gov/pubmed/25648511
- Xie R, Liu M. Relationship Between Non-Alcoholic Fatty Liver Disease and Degree of Hepatic Steatosis and Bone Mineral Density. Frontiers in endocrinology. 2022;13:857110. doi:10.3389/fendo.2022.857110. https://www.ncbi.nlm.nih.gov/pubmed/35360054
- Zhai T, Chen Q, Xu J, Jia X, Xia P. Prevalence and Trends in Low Bone Density, Osteopenia and Osteoporosis in U.S. Adults With Non-Alcoholic Fatty Liver Disease, 2005-2014. Frontiers in endocrinology. 2021;12:825448. doi:10.3389/fendo.2021.825448. https://www.ncbi.nlm.nih.gov/pubmed/35126317
- Zhu X, Yan H, Chang X, et al. Association between non-alcoholic fatty liver disease-associated hepatic fibrosis and bone mineral density in postmenopausal women with type 2 diabetes or impaired glucose regulation. BMJ Open Diabetes Res Care. Aug 2020;8(1)doi:10.1136/bmjdrc-2019-000999. https://www.ncbi.nlm.nih.gov/pubmed/32759166
- Ciardullo S, Muraca E, Zerbini F, Manzoni G, Perseghin G. NAFLD and Liver Fibrosis Are Not Associated With Reduced Femoral Bone Mineral Density in the General US Population. J Clin Endocrinol Metab. Jul 13 2021;106(8):e2856-e2865. doi:10.1210/clinem/dgab262. https://www.ncbi.nlm.nih.gov/pubmed/33878156
- Sung J, Ryu S, Song YM, Cheong HK. Relationship Between Non-alcoholic Fatty Liver Disease and Decreased Bone Mineral Density: A Retrospective Cohort Study in Korea. J Prev Med Public Health. Sep 2020;53(5):342-352. doi:10.3961/jpmph.20.089. https://www.ncbi.nlm.nih.gov/pubmed/33070506
- Emamat H, Ghalandari H, Totmaj AS, Tangestani H, Hekmatdoost A. Calcium to magnesium intake ratio and non-alcoholic fatty liver disease development: a case-control study. BMC endocrine disorders. Mar 18 2021;21(1):51. doi:10.1186/s12902-021-00721-w. https://www.ncbi.nlm.nih.gov/pubmed/33736626
- Li W, Zhu X, Song Y, et al. Intakes of magnesium, calcium and risk of fatty liver disease and prediabetes. Public health nutrition. Aug 2018;21(11):2088-2095. doi:10.1017/S1368980018000642. https://www.ncbi.nlm.nih.gov/pubmed/29607802
- Lu L, Chen C, Li Y, et al. Magnesium intake is inversely associated with risk of non-alcoholic fatty liver disease among American adults. European journal of nutrition. Apr 2022;61(3):1245-1254. doi:10.1007/s00394-021-02732-8. https://www.ncbi.nlm.nih.gov/pubmed/34741649
- Eshraghian A, Nikeghbalian S, Geramizadeh B, Malek-Hosseini SA. Serum magnesium concentration is independently associated with non-alcoholic fatty liver and non-alcoholic steatohepatitis. United European Gastroenterol J. Feb 2018;6(1):97-103. doi:10.1177/2050640617707863. https://www.ncbi.nlm.nih.gov/pubmed/29435319
- Karandish M, Tamimi M, Shayesteh AA, Haghighizadeh MH, Jalali MT. The effect of magnesium supplementation and weight loss on liver enzymes in patients with nonalcoholic fatty liver disease. Journal of research in medical sciences : the official journal of Isfahan University of Medical Sciences . Jul 2013;18(7):573-9. https://www.ncbi.nlm.nih.gov/pubmed/24516489
- Akhavan Rezayat A, Dadgar Moghadam M, Ghasemi Nour M, et al. Association between smoking and non-alcoholic fatty liver disease: A systematic review and meta-analysis. SAGE open medicine. 2018;6:2050312117745223. doi:10.1177/2050312117745223. https://www.ncbi.nlm.nih.gov/pubmed/29399359
- Bruneau A, Hundertmark J, Guillot A, Tacke F. Molecular and Cellular Mediators of the Gut-Liver Axis in the Progression of Liver Diseases. Front Med (Lausanne). 2021;8:725390. doi:10.3389/fmed.2021.725390. https://www.ncbi.nlm.nih.gov/pubmed/34650994
- Wijarnpreecha K, Lou S, Watthanasuntorn K, et al. Small intestinal bacterial overgrowth and nonalcoholic fatty liver disease: a systematic review and meta-analysis. European journal of gastroenterology & hepatology. May 2020;32(5):601-608. doi:10.1097/MEG.0000000000001541. https://www.ncbi.nlm.nih.gov/pubmed/31567712
- Purssell H, Whorwell PJ, Athwal VS, Vasant DH. Non-alcoholic fatty liver disease in irritable bowel syndrome: More than a coincidence? World J Hepatol. Dec 27 2021;13(12):1816-1827. doi:10.4254/wjh.v13.i12.1816. https://www.ncbi.nlm.nih.gov/pubmed/35069992
- Mikolasevic I, Poropat G, Filipec Kanizaj T, et al. Association between Gastroesophageal Reflux Disease and Elastographic Parameters of Liver Steatosis and Fibrosis: Controlled Attenuation Parameter and Liver Stiffness Measurements. Can J Gastroenterol Hepatol. 2021;2021:6670065. doi:10.1155/2021/6670065. https://www.ncbi.nlm.nih.gov/pubmed/33688490
- Ghoshal UC, Baba CS, Ghoshal U, et al. Low-grade small intestinal bacterial overgrowth is common in patients with non-alcoholic steatohepatitis on quantitative jejunal aspirate culture. Indian journal of gastroenterology : official journal of the Indian Society of Gastroenterology . Sep 2017;36(5):390-399. doi:10.1007/s12664-017-0797-6. https://www.ncbi.nlm.nih.gov/pubmed/29034439
- Mikolasevic I, Delija B, Mijic A, et al. Small intestinal bacterial overgrowth and non-alcoholic fatty liver disease diagnosed by transient elastography and liver biopsy. International journal of clinical practice. Apr 2021;75(4):e13947. doi:10.1111/ijcp.13947. https://www.ncbi.nlm.nih.gov/pubmed/33406286
- Wei L, Ding HG. Relationship between Helicobacter pylori infection and nonalcoholic fatty liver disease: What should we expect from a meta-analysis? Medicine. Aug 6 2021;100(31):e26706. doi:10.1097/MD.0000000000026706. https://www.ncbi.nlm.nih.gov/pubmed/34397807
- Takakura W, Pimentel M. Small Intestinal Bacterial Overgrowth and Irritable Bowel Syndrome - An Update. Frontiers in psychiatry. 2020;11:664. doi:10.3389/fpsyt.2020.00664. https://www.ncbi.nlm.nih.gov/pubmed/32754068
- Ding JH, Jin Z, Yang XX, et al. Role of gut microbiota via the gut-liver-brain axis in digestive diseases. World J Gastroenterol. Oct 28 2020;26(40):6141-6162. doi:10.3748/wjg.v26.i40.6141. https://www.ncbi.nlm.nih.gov/pubmed/33177790
- Farre R, Fiorani M, Abdu Rahiman S, Matteoli G. Intestinal Permeability, Inflammation and the Role of Nutrients. Nutrients. Apr 23 2020;12(4)doi:10.3390/nu12041185. https://www.ncbi.nlm.nih.gov/pubmed/32340206
- Chen J, Vitetta L. Gut Microbiota Metabolites in NAFLD Pathogenesis and Therapeutic Implications. International journal of molecular sciences. Jul 23 2020;21(15)doi:10.3390/ijms21155214. https://www.ncbi.nlm.nih.gov/pubmed/32717871
- Liptak R, Gromova B, Gardlik R. Fecal Microbiota Transplantation as a Tool for Therapeutic Modulation of Non-gastrointestinal Disorders. Front Med (Lausanne). 2021;8:665520. doi:10.3389/fmed.2021.665520. https://www.ncbi.nlm.nih.gov/pubmed/34557498
- Malnick SDH, Fisher D, Somin M, Neuman MG. Treating the Metabolic Syndrome by Fecal Transplantation-Current Status. Biology. May 20 2021;10(5)doi:10.3390/biology10050447. https://www.ncbi.nlm.nih.gov/pubmed/34065241
- Craven L, Rahman A, Nair Parvathy S, et al. Allogenic Fecal Microbiota Transplantation in Patients With Nonalcoholic Fatty Liver Disease Improves Abnormal Small Intestinal Permeability: A Randomized Control Trial. The American journal of gastroenterology. Jul 2020;115(7):1055-1065. doi:10.14309/ajg.0000000000000661. https://www.ncbi.nlm.nih.gov/pubmed/32618656
- Khan A, Ding Z, Ishaq M, et al. Understanding the Effects of Gut Microbiota Dysbiosis on Nonalcoholic Fatty Liver Disease and the Possible Probiotics Role: Recent Updates. International journal of biological sciences . 2021;17(3):818-833. doi:10.7150/ijbs.56214. https://www.ncbi.nlm.nih.gov/pubmed/33767591
- Mitsinikos T, Mrowczynski-Hernandez P, Kohli R. Pediatric Nonalcoholic Fatty Liver Disease. Pediatric clinics of North America. Dec 2021;68(6):1309-1320. doi:10.1016/j.pcl.2021.07.013. https://www.ncbi.nlm.nih.gov/pubmed/34736591
- Lee C, Kim J, Jung Y. Potential Therapeutic Application of Estrogen in Gender Disparity of Nonalcoholic Fatty Liver Disease/Nonalcoholic Steatohepatitis. Cells. Oct 15 2019;8(10)doi:10.3390/cells8101259. https://www.ncbi.nlm.nih.gov/pubmed/31619023
- DiStefano JK. NAFLD and NASH in Postmenopausal Women: Implications for Diagnosis and Treatment. Endocrinology. Oct 1 2020;161(10)doi:10.1210/endocr/bqaa134. https://www.ncbi.nlm.nih.gov/pubmed/32776116
- Moran-Costoya A, Proenza AM, Gianotti M, Llado I, Valle A. Sex Differences in Nonalcoholic Fatty Liver Disease: Estrogen Influence on the Liver-Adipose Tissue Crosstalk. Antioxid Redox Signal. Sep 20 2021;35(9):753-774. doi:10.1089/ars.2021.0044. https://www.ncbi.nlm.nih.gov/pubmed/33736456
- Bonacini M, Kassamali F, Kari S, Lopez Barrera N, Kohla M. Racial differences in prevalence and severity of non-alcoholic fatty liver disease. World J Hepatol. Jul 27 2021;13(7):763-773. doi:10.4254/wjh.v13.i7.763. https://www.ncbi.nlm.nih.gov/pubmed/34367497
- Spremovic Radenovic S, Pupovac M, Andjic M, et al. Prevalence, Risk Factors, and Pathophysiology of Nonalcoholic Fatty Liver Disease (NAFLD) in Women with Polycystic Ovary Syndrome (PCOS). Biomedicines. Jan 7 2022;10(1)doi:10.3390/biomedicines10010131. https://www.ncbi.nlm.nih.gov/pubmed/35052811
- Zeng X, Li B, Zou Y. The relationship between non-alcoholic fatty liver disease and hypothyroidism: A systematic review and meta-analysis. Medicine. Apr 30 2021;100(17):e25738. doi:10.1097/MD.0000000000025738. https://www.ncbi.nlm.nih.gov/pubmed/33907168
- Shea S, Lionis C, Kite C, et al. Non-Alcoholic Fatty Liver Disease (NAFLD) and Potential Links to Depression, Anxiety, and Chronic Stress. Biomedicines. Nov 16 2021;9(11)doi:10.3390/biomedicines9111697. https://www.ncbi.nlm.nih.gov/pubmed/34829926
- Labenz C, Huber Y, Michel M, et al. Nonalcoholic Fatty Liver Disease Increases the Risk of Anxiety and Depression. Hepatology communications. Sep 2020;4(9):1293-1301. doi:10.1002/hep4.1541. https://www.ncbi.nlm.nih.gov/pubmed/32923833
- Sukahri S, Mohamed Shah FZ, Ismail AI, et al. Significantly higher atherosclerosis risks in patients with obstructive sleep apnea and non-alcoholic fatty liver disease. PLoS One. 2021;16(6):e0253298. doi:10.1371/journal.pone.0253298. https://www.ncbi.nlm.nih.gov/pubmed/34191823
- Jawa HA, Khatib H, Alzahrani N, et al. Nonalcoholic Fatty Liver Disease and Fibrosis Risk in Patients With Obstructive Sleep Apnea: A Retrospective Analysis. Cureus. Feb 28 2021;13(2):e13623. doi:10.7759/cureus.13623. https://www.ncbi.nlm.nih.gov/pubmed/33816022
- Bahr K, Simon P, Leggewie B, Gouveris H, Schattenberg J. The Snoring Index Identifies Risk of Non-Alcoholic Fatty Liver Disease in Patients with Obstructive Sleep Apnea Syndrome. Biology. Dec 22 2021;11(1)doi:10.3390/biology11010010. https://www.ncbi.nlm.nih.gov/pubmed/35053008
- Jin S, Jiang S, Hu A. Association between obstructive sleep apnea and non-alcoholic fatty liver disease: a systematic review and meta-analysis. Sleep & breathing = Schlaf & Atmung. Sep 2018;22(3):841-851. doi:10.1007/s11325-018-1625-7. https://www.ncbi.nlm.nih.gov/pubmed/29335916
- Chung GE, Cho EJ, Yoo JJ, et al. Nonalcoholic fatty liver disease is associated with the development of obstructive sleep apnea. Sci Rep. Jun 29 2021;11(1):13473. doi:10.1038/s41598-021-92703-0. https://www.ncbi.nlm.nih.gov/pubmed/34188101
- Hatasa M, Yoshida S, Takahashi H, et al. Relationship between NAFLD and Periodontal Disease from the View of Clinical and Basic Research, and Immunological Response. International journal of molecular sciences. Apr 2 2021;22(7)doi:10.3390/ijms22073728. https://www.ncbi.nlm.nih.gov/pubmed/33918456
- Wijarnpreecha K, Panjawatanan P, Cheungpasitporn W, et al. The Association between Periodontitis and Nonalcoholic Fatty Liver Disease: A Systematic Review and Meta-analysis. Journal of gastrointestinal and liver diseases : JGLD. Jun 3 2020;29(2):211-217. doi:10.15403/jgld-841. https://www.ncbi.nlm.nih.gov/pubmed/32530988
- Cespiati A, Meroni M, Lombardi R, Oberti G, Dongiovanni P, Fracanzani AL. Impact of Sarcopenia and Myosteatosis in Non-Cirrhotic Stages of Liver Diseases: Similarities and Differences across Aetiologies and Possible Therapeutic Strategies. Biomedicines. Jan 16 2022;10(1)doi:10.3390/biomedicines10010182. https://www.ncbi.nlm.nih.gov/pubmed/35052859
- Cheung A, Ahmed A. Nonalcoholic Fatty Liver Disease and Chronic Kidney Disease: A Review of Links and Risks. Clin Exp Gastroenterol. 2021;14:457-465. doi:10.2147/CEG.S226130. https://www.ncbi.nlm.nih.gov/pubmed/34819740
- Wang TY, Wang RF, Bu ZY, et al. Association of metabolic dysfunction-associated fatty liver disease with kidney disease. Nat Rev Nephrol. Apr 2022;18(4):259-268. doi:10.1038/s41581-021-00519-y. https://www.ncbi.nlm.nih.gov/pubmed/35013596
- Mantovani A, Petracca G, Beatrice G, et al. Non-alcoholic fatty liver disease and risk of incident chronic kidney disease: an updated meta-analysis. Gut. Jan 2022;71(1):156-162. doi:10.1136/gutjnl-2020-323082. https://www.ncbi.nlm.nih.gov/pubmed/33303564
- Yang R, Shang J, Zhou Y, Liu W, Tian Y, Shang H. Effects of probiotics on nonalcoholic fatty liver disease: a systematic review and meta-analysis. Expert review of gastroenterology & hepatology. Dec 2021;15(12):1401-1409. doi:10.1080/17474124.2022.2016391. https://www.ncbi.nlm.nih.gov/pubmed/34877910
- Eslamparast T, Poustchi H, Zamani F, Sharafkhah M, Malekzadeh R, Hekmatdoost A. Synbiotic supplementation in nonalcoholic fatty liver disease: a randomized, double-blind, placebo-controlled pilot study. Am J Clin Nutr. Mar 2014;99(3):535-42. doi:10.3945/ajcn.113.068890. https://www.ncbi.nlm.nih.gov/pubmed/24401715
- Ahn SB, Jun DW, Kang BK, Lim JH, Lim S, Chung MJ. Randomized, Double-blind, Placebo-controlled Study of a Multispecies Probiotic Mixture in Nonalcoholic Fatty Liver Disease. Sci Rep. Apr 5 2019;9(1):5688. doi:10.1038/s41598-019-42059-3. https://www.ncbi.nlm.nih.gov/pubmed/30952918
- Ferolla SM, Couto CA, Costa-Silva L, et al. Beneficial Effect of Synbiotic Supplementation on Hepatic Steatosis and Anthropometric Parameters, But Not on Gut Permeability in a Population with Nonalcoholic Steatohepatitis. Nutrients. Jun 28 2016;8(7)doi:10.3390/nu8070397. https://www.ncbi.nlm.nih.gov/pubmed/27367724
- Kobyliak N, Abenavoli L, Mykhalchyshyn G, et al. A Multi-strain Probiotic Reduces the Fatty Liver Index, Cytokines and Aminotransferase levels in NAFLD Patients: Evidence from a Randomized Clinical Trial. Journal of gastrointestinal and liver diseases : JGLD. Mar 2018;27(1):41-49. doi:10.15403/jgld.2014.1121.271.kby. https://www.ncbi.nlm.nih.gov/pubmed/29557414
- Behrouz V, Aryaeian N, Zahedi MJ, Jazayeri S. Effects of probiotic and prebiotic supplementation on metabolic parameters, liver aminotransferases, and systemic inflammation in nonalcoholic fatty liver disease: A randomized clinical trial. Journal of food science. Oct 2020;85(10):3611-3617. doi:10.1111/1750-3841.15367. https://www.ncbi.nlm.nih.gov/pubmed/32885440
- Aller R, De Luis DA, Izaola O, et al. Effect of a probiotic on liver aminotransferases in nonalcoholic fatty liver disease patients: a double blind randomized clinical trial. European review for medical and pharmacological sciences. Sep 2011;15(9):1090-5. https://www.ncbi.nlm.nih.gov/pubmed/22013734
- Abhari K, Saadati S, Yari Z, et al. The effects of Bacillus coagulans supplementation in patients with non-alcoholic fatty liver disease: A randomized, placebo-controlled, clinical trial. Clinical nutrition ESPEN. Oct 2020;39:53-60. doi:10.1016/j.clnesp.2020.06.020. https://www.ncbi.nlm.nih.gov/pubmed/32859329
- Mofidi F, Poustchi H, Yari Z, et al. Synbiotic supplementation in lean patients with non-alcoholic fatty liver disease: a pilot, randomised, double-blind, placebo-controlled, clinical trial. The British journal of nutrition. Mar 2017;117(5):662-668. doi:10.1017/S0007114517000204. https://www.ncbi.nlm.nih.gov/pubmed/28345499
- Mohamad Nor MH, Ayob N, Mokhtar NM, et al. The Effect of Probiotics (MCP((R)) BCMC((R)) Strains) on Hepatic Steatosis, Small Intestinal Mucosal Immune Function, and Intestinal Barrier in Patients with Non-Alcoholic Fatty Liver Disease. Nutrients. Sep 14 2021;13(9)doi:10.3390/nu13093192. https://www.ncbi.nlm.nih.gov/pubmed/34579068
- Chong PL, Laight D, Aspinall RJ, Higginson A, Cummings MH. A randomised placebo controlled trial of VSL#3((R)) probiotic on biomarkers of cardiovascular risk and liver injury in non-alcoholic fatty liver disease. BMC gastroenterology. Apr 1 2021;21(1):144. doi:10.1186/s12876-021-01660-5. https://www.ncbi.nlm.nih.gov/pubmed/33794784
- Vadarlis A, Antza C, Bakaloudi DR, et al. Systematic review with meta-analysis: The effect of vitamin E supplementation in adult patients with non-alcoholic fatty liver disease. Journal of gastroenterology and hepatology. Feb 2021;36(2):311-319. doi:10.1111/jgh.15221. https://www.ncbi.nlm.nih.gov/pubmed/32810309
- Amanullah I, Khan YH, Anwar I, Gulzar A, Mallhi TH, Raja AA. Effect of vitamin E in non-alcoholic fatty liver disease: a systematic review and meta-analysis of randomised controlled trials. Postgrad Med J. Nov 2019;95(1129):601-611. doi:10.1136/postgradmedj-2018-136364. https://www.ncbi.nlm.nih.gov/pubmed/31434683
- Majzoub AM, Nayfeh T, Barnard A, et al. Systematic review with network meta-analysis: comparative efficacy of pharmacologic therapies for fibrosis improvement and resolution of NASH. Alimentary pharmacology & therapeutics. Oct 2021;54(7):880-889. doi:10.1111/apt.16583. https://www.ncbi.nlm.nih.gov/pubmed/34435378
- Kedarisetty CK, Bhardwaj A, Kumar G, et al. Efficacy of combining pentoxiphylline and vitamin E versus vitamin E alone in non-alcoholic steatohepatitis- A randomized pilot study. Indian journal of gastroenterology : official journal of the Indian Society of Gastroenterology . Feb 2021;40(1):41-49. doi:10.1007/s12664-020-01131-x. https://www.ncbi.nlm.nih.gov/pubmed/33772456
- Polyzos SA, Kountouras J, Mantzoros CS, Polymerou V, Katsinelos P. Effects of combined low-dose spironolactone plus vitamin E vs vitamin E monotherapy on insulin resistance, non-invasive indices of steatosis and fibrosis, and adipokine levels in non-alcoholic fatty liver disease: a randomized controlled trial. Diabetes, obesity & metabolism. Dec 2017;19(12):1805-1809. doi:10.1111/dom.12989. https://www.ncbi.nlm.nih.gov/pubmed/28452101
- Banini BA, Cazanave SC, Yates KP, et al. Haptoglobin 2 Allele is Associated With Histologic Response to Vitamin E in Subjects With Nonalcoholic Steatohepatitis. Journal of clinical gastroenterology. Nov/Dec 2019;53(10):750-758. doi:10.1097/MCG.0000000000001142. https://www.ncbi.nlm.nih.gov/pubmed/30586008
- Pervez MA, Khan DA, Slehria AUR, Ijaz A. Delta-tocotrienol supplementation improves biochemical markers of hepatocellular injury and steatosis in patients with nonalcoholic fatty liver disease: A randomized, placebo-controlled trial. Complementary therapies in medicine. Aug 2020;52:102494. doi:10.1016/j.ctim.2020.102494. https://www.ncbi.nlm.nih.gov/pubmed/32951743
- Magosso E, Ansari MA, Gopalan Y, et al. Tocotrienols for normalisation of hepatic echogenic response in nonalcoholic fatty liver: a randomised placebo-controlled clinical trial. Nutr J. Dec 27 2013;12(1):166. doi:10.1186/1475-2891-12-166. https://www.ncbi.nlm.nih.gov/pubmed/24373555
- Abenavoli L, Izzo AA, Milic N, Cicala C, Santini A, Capasso R. Milk thistle (Silybum marianum): A concise overview on its chemistry, pharmacological, and nutraceutical uses in liver diseases. Phytother Res. Nov 2018;32(11):2202-2213. doi:10.1002/ptr.6171. https://www.ncbi.nlm.nih.gov/pubmed/30080294
- Kalopitas G, Antza C, Doundoulakis I, et al. Impact of Silymarin in individuals with nonalcoholic fatty liver disease: A systematic review and meta-analysis. Nutrition (Burbank, Los Angeles County, Calif). Mar 2021;83:111092. doi:10.1016/j.nut.2020.111092. https://www.ncbi.nlm.nih.gov/pubmed/33418491
- Zhong S, Fan Y, Yan Q, et al. The therapeutic effect of silymarin in the treatment of nonalcoholic fatty disease: A meta-analysis (PRISMA) of randomized control trials. Medicine. Dec 2017;96(49):e9061. doi:10.1097/MD.0000000000009061. https://www.ncbi.nlm.nih.gov/pubmed/29245314
- Wah Kheong C, Nik Mustapha NR, Mahadeva S. A Randomized Trial of Silymarin for the Treatment of Nonalcoholic Steatohepatitis. Clinical gastroenterology and hepatology : the official clinical practice journal of the American Gastroenterological Association . Dec 2017;15(12):1940-1949 e8. doi:10.1016/j.cgh.2017.04.016. https://www.ncbi.nlm.nih.gov/pubmed/28419855
- Sorrentino G, Crispino P, Coppola D, De Stefano G. Efficacy of lifestyle changes in subjects with non-alcoholic liver steatosis and metabolic syndrome may be improved with an antioxidant nutraceutical: a controlled clinical study. Drugs in R&D. Mar 2015;15(1):21-5. doi:10.1007/s40268-015-0084-x. https://www.ncbi.nlm.nih.gov/pubmed/25732561
- Aller R, Izaola O, Gomez S, et al. Effect of silymarin plus vitamin E in patients with non-alcoholic fatty liver disease. A randomized clinical pilot study. European review for medical and pharmacological sciences. Aug 2015;19(16):3118-24. https://www.ncbi.nlm.nih.gov/pubmed/26367736
- Poulos JE, Kalogerinis PT, Milanov V, Kalogerinis CT, Poulos EJ. The Effects of Vitamin E, Silymarin and Carnitine on the Metabolic Abnormalities Associated with Nonalcoholic Liver Disease. Journal of dietary supplements. Jan 25 2022;19(3):287-302. doi:10.1080/19390211.2021.1874587. https://www.ncbi.nlm.nih.gov/pubmed/33491528
- Curcio A, Romano A, Cuozzo S, et al. Silymarin in Combination with Vitamin C, Vitamin E, Coenzyme Q10 and Selenomethionine to Improve Liver Enzymes and Blood Lipid Profile in NAFLD Patients. Medicina (Kaunas, Lithuania). Oct 17 2020;56(10)doi:10.3390/medicina56100544. https://www.ncbi.nlm.nih.gov/pubmed/33080906
- Dumitru E, Condur L, Alexandrescu L, et al. Triple Antioxidant Therapy, an Alternative for Patients with Chronic Liver Disease - A Prospective Multicenter Interventional Study. Maedica (Bucur). Dec 2020;15(4):433-439. doi:10.26574/maedica.2020.15.4.433. https://www.ncbi.nlm.nih.gov/pubmed/33603899
- Federico A, Dallio M, Masarone M, et al. Evaluation of the Effect Derived from Silybin with Vitamin D and Vitamin E Administration on Clinical, Metabolic, Endothelial Dysfunction, Oxidative Stress Parameters, and Serological Worsening Markers in Nonalcoholic Fatty Liver Disease Patients. Oxid Med Cell Longev. 2019;2019:8742075. doi:10.1155/2019/8742075. https://www.ncbi.nlm.nih.gov/pubmed/31737175
- Lv DD, Wang YJ, Wang ML, et al. Effect of silibinin capsules combined with lifestyle modification on hepatic steatosis in patients with chronic hepatitis B. Sci Rep. Jan 12 2021;11(1):655. doi:10.1038/s41598-020-80709-z. https://www.ncbi.nlm.nih.gov/pubmed/33436935
- Neag MA, Mocan A, Echeverria J, et al. Berberine: Botanical Occurrence, Traditional Uses, Extraction Methods, and Relevance in Cardiovascular, Metabolic, Hepatic, and Renal Disorders. Frontiers in pharmacology . 2018;9:557. doi:10.3389/fphar.2018.00557. https://www.ncbi.nlm.nih.gov/pubmed/30186157
- Ren S, Ma X, Wang R, et al. Preclinical Evidence of Berberine on Non-Alcoholic Fatty Liver Disease: A Systematic Review and Meta-Analysis of Animal Studies. Frontiers in pharmacology. 2021;12:742465. doi:10.3389/fphar.2021.742465. https://www.ncbi.nlm.nih.gov/pubmed/34566663
- Wei X, Wang C, Hao S, Song H, Yang L. The Therapeutic Effect of Berberine in the Treatment of Nonalcoholic Fatty Liver Disease: A Meta-Analysis. Evidence-based complementary and alternative medicine : eCAM. 2016;2016:3593951. doi:10.1155/2016/3593951. https://www.ncbi.nlm.nih.gov/pubmed/27446224
- Chang X, Wang Z, Zhang J, et al. Lipid profiling of the therapeutic effects of berberine in patients with nonalcoholic fatty liver disease. Journal of translational medicine. Sep 15 2016;14:266. doi:10.1186/s12967-016-0982-x. https://www.ncbi.nlm.nih.gov/pubmed/27629750
- Harrison SA, Gunn N, Neff GW, et al. A phase 2, proof of concept, randomised controlled trial of berberine ursodeoxycholate in patients with presumed non-alcoholic steatohepatitis and type 2 diabetes. Nature communications. Sep 17 2021;12(1):5503. doi:10.1038/s41467-021-25701-5. https://www.ncbi.nlm.nih.gov/pubmed/34535644
- Kysenius K, Brunello CA, Huttunen HJ. Mitochondria and NMDA Receptor-Dependent Toxicity of Berberine Sensitizes Neurons to Glutamate and Rotenone Injury. PLOS ONE. 2014;9(9):e107129. doi:10.1371/journal.pone.0107129. https://doi.org/10.1371/journal.pone.0107129
- Mikeš V, Yaguzhinskij LS. Interaction of fluorescent berberine alkyl derivatives with respiratory chain of rat liver mitochondria. Journal of Bioenergetics and Biomembranes. 1985/02/01 1985;17(1):23-32. doi:10.1007/BF00744986. https://doi.org/10.1007/BF00744986
- Mikeš V, Dadák V. Berberine derivatives as cationic fluorescent probes for the investigation of the energized state of mitochondria. Biochimica et Biophysica Acta (BBA) - Bioenergetics. 1983/05/27/ 1983;723(2):231-239. doi:https://doi.org/10.1016/0005-2728(83)90122-6. https://www.sciencedirect.com/science/article/pii/0005272883901226
- Jabczyk M, Nowak J, Hudzik B, Zubelewicz-Szkodzinska B. Curcumin in Metabolic Health and Disease. Nutrients. Dec 11 2021;13(12)doi:10.3390/nu13124440. https://www.ncbi.nlm.nih.gov/pubmed/34959992
- Jalali M, Mahmoodi M, Mosallanezhad Z, Jalali R, Imanieh MH, Moosavian SP. The effects of curcumin supplementation on liver function, metabolic profile and body composition in patients with non-alcoholic fatty liver disease: A systematic review and meta-analysis of randomized controlled trials. Complementary therapies in medicine. Jan 2020;48:102283. doi:10.1016/j.ctim.2019.102283. https://www.ncbi.nlm.nih.gov/pubmed/31987259
- Baziar N, Parohan M. The effects of curcumin supplementation on body mass index, body weight, and waist circumference in patients with nonalcoholic fatty liver disease: A systematic review and dose-response meta-analysis of randomized controlled trials. Phytother Res. Mar 2020;34(3):464-474. doi:10.1002/ptr.6542. https://www.ncbi.nlm.nih.gov/pubmed/31799714
- Wei Z, Liu N, Tantai X, et al. The effects of curcumin on the metabolic parameters of non-alcoholic fatty liver disease: a meta-analysis of randomized controlled trials. Hepatol Int. May 2019;13(3):302-313. doi:10.1007/s12072-018-9910-x. https://www.ncbi.nlm.nih.gov/pubmed/30446932
- Goodarzi R, Sabzian K, Shishehbor F, Mansoori A. Does turmeric/curcumin supplementation improve serum alanine aminotransferase and aspartate aminotransferase levels in patients with nonalcoholic fatty liver disease? A systematic review and meta-analysis of randomized controlled trials. Phytother Res. Mar 2019;33(3):561-570. doi:10.1002/ptr.6270. https://www.ncbi.nlm.nih.gov/pubmed/30653773
- Saadati S, Sadeghi A, Mansour A, et al. Curcumin and inflammation in non-alcoholic fatty liver disease: a randomized, placebo controlled clinical trial. BMC gastroenterology. Jul 25 2019;19(1):133. doi:10.1186/s12876-019-1055-4. https://www.ncbi.nlm.nih.gov/pubmed/31345163
- Saadati S, Hatami B, Yari Z, et al. The effects of curcumin supplementation on liver enzymes, lipid profile, glucose homeostasis, and hepatic steatosis and fibrosis in patients with non-alcoholic fatty liver disease. European journal of clinical nutrition. Mar 2019;73(3):441-449. doi:10.1038/s41430-018-0382-9. https://www.ncbi.nlm.nih.gov/pubmed/30610213
- Jarhahzadeh M, Alavinejad P, Farsi F, Husain D, Rezazadeh A. The effect of turmeric on lipid profile, malondialdehyde, liver echogenicity and enzymes among patients with nonalcoholic fatty liver disease: a randomized double blind clinical trial. Diabetol Metab Syndr. Oct 18 2021;13(1):112. doi:10.1186/s13098-021-00731-7. https://www.ncbi.nlm.nih.gov/pubmed/34663438
- Lopresti AL. The Problem of Curcumin and Its Bioavailability: Could Its Gastrointestinal Influence Contribute to Its Overall Health-Enhancing Effects? Adv Nutr. Jan 1 2018;9(1):41-50. doi:10.1093/advances/nmx011. https://www.ncbi.nlm.nih.gov/pubmed/29438458
- Witika BA, Makoni PA, Matafwali SK, Mweetwa LL, Shandele GC, Walker RB. Enhancement of Biological and Pharmacological Properties of an Encapsulated Polyphenol: Curcumin. Molecules (Basel, Switzerland). Jul 13 2021;26(14)doi:10.3390/molecules26144244. https://www.ncbi.nlm.nih.gov/pubmed/34299519
- Panahi Y, Valizadegan G, Ahamdi N, Ganjali S, Majeed M, Sahebkar A. Curcuminoids plus piperine improve nonalcoholic fatty liver disease: A clinical trial. Journal of cellular biochemistry. Sep 2019;120(9):15989-15996. doi:10.1002/jcb.28877. https://www.ncbi.nlm.nih.gov/pubmed/31168845
- Mirhafez SR, Dehabeh M, Hariri M, et al. Curcumin and Piperine Combination for the Treatment of Patients with Non-alcoholic Fatty Liver Disease: A Double-Blind Randomized Placebo-Controlled Trial. Adv Exp Med Biol . 2021;1328:11-19. doi:10.1007/978-3-030-73234-9_2. https://www.ncbi.nlm.nih.gov/pubmed/34981468
- Saberi-Karimian M, Keshvari M, Ghayour-Mobarhan M, et al. Effects of curcuminoids on inflammatory status in patients with non-alcoholic fatty liver disease: A randomized controlled trial. Complementary therapies in medicine. Mar 2020;49:102322. doi:10.1016/j.ctim.2020.102322. https://www.ncbi.nlm.nih.gov/pubmed/32147075
- Mirhafez SR, Azimi-Nezhad M, Dehabeh M, et al. The Effect of Curcumin Phytosome on the Treatment of Patients with Non-alcoholic Fatty Liver Disease: A Double-Blind, Randomized, Placebo-Controlled Trial. Adv Exp Med Biol. 2021;1308:25-35. doi:10.1007/978-3-030-64872-5_3. https://www.ncbi.nlm.nih.gov/pubmed/33861434
- Cicero AFG, Sahebkar A, Fogacci F, Bove M, Giovannini M, Borghi C. Effects of phytosomal curcumin on anthropometric parameters, insulin resistance, cortisolemia and non-alcoholic fatty liver disease indices: a double-blind, placebo-controlled clinical trial. European journal of nutrition. Mar 2020;59(2):477-483. doi:10.1007/s00394-019-01916-7. https://www.ncbi.nlm.nih.gov/pubmed/30796508
- Jazayeri-Tehrani SA, Rezayat SM, Mansouri S, et al. Nano-curcumin improves glucose indices, lipids, inflammation, and Nesfatin in overweight and obese patients with non-alcoholic fatty liver disease (NAFLD): a double-blind randomized placebo-controlled clinical trial. Nutr Metab (Lond). 2019;16:8. doi:10.1186/s12986-019-0331-1. https://www.ncbi.nlm.nih.gov/pubmed/30705687
- Novais AA, Chuffa LGA, Zuccari D, Reiter RJ. Exosomes and Melatonin: Where Their Destinies Intersect. Front Immunol. 2021;12:692022. doi:10.3389/fimmu.2021.692022. https://www.ncbi.nlm.nih.gov/pubmed/34177952
- Abdi S, Abbasinazari M, Ataei S, Khanzadeh-Moghaddam N, Keshvari N. Benefits and Risks of Melatonin in Hepatic and Pancreatic Disorders; A Review of Clinical Evidences. Iranian journal of pharmaceutical research : IJPR. Summer 2021;20(3):102-109. doi:10.22037/ijpr.2020.114477.14872. https://www.ncbi.nlm.nih.gov/pubmed/34903973
- Mansoori A, Salimi Z, Hosseini SA, et al. The effect of melatonin supplementation on liver indices in patients with non-alcoholic fatty liver disease: A systematic review and meta-analysis of randomized clinical trials. Complementary therapies in medicine. Aug 2020;52:102398. doi:10.1016/j.ctim.2020.102398. https://www.ncbi.nlm.nih.gov/pubmed/32951697
- Akhavan Rezayat A, Ghasemi Nour M, Bondarsahebi Y, et al. The effects of melatonin therapy on the treatment of patients with Non-alcoholic steatohepatitis: A systematic review and Meta-analysis on clinical trial studies. European journal of pharmacology. Aug 15 2021;905:174154. doi:10.1016/j.ejphar.2021.174154. https://www.ncbi.nlm.nih.gov/pubmed/34058202
- Bahrami M, Cheraghpour M, Jafarirad S, et al. The effect of melatonin on treatment of patients with non-alcoholic fatty liver disease: a randomized double blind clinical trial. Complementary therapies in medicine. Aug 2020;52:102452. doi:10.1016/j.ctim.2020.102452. https://www.ncbi.nlm.nih.gov/pubmed/32951715
- Gonciarz M, Gonciarz Z, Bielanski W, et al. The effects of long-term melatonin treatment on plasma liver enzymes levels and plasma concentrations of lipids and melatonin in patients with nonalcoholic steatohepatitis: a pilot study. Journal of physiology and pharmacology : an official journal of the Polish Physiological Society . Feb 2012;63(1):35-40. https://www.ncbi.nlm.nih.gov/pubmed/22460459
- Gonciarz M, Gonciarz Z, Bielanski W, et al. The pilot study of 3-month course of melatonin treatment of patients with nonalcoholic steatohepatitis: effect on plasma levels of liver enzymes, lipids and melatonin. Journal of physiology and pharmacology : an official journal of the Polish Physiological Society . Dec 2010;61(6):705-10. https://www.ncbi.nlm.nih.gov/pubmed/21224501
- Celinski K, Konturek PC, Slomka M, et al. Effects of treatment with melatonin and tryptophan on liver enzymes, parameters of fat metabolism and plasma levels of cytokines in patients with non-alcoholic fatty liver disease--14 months follow up. Journal of physiology and pharmacology : an official journal of the Polish Physiological Society . Feb 2014;65(1):75-82. https://www.ncbi.nlm.nih.gov/pubmed/24622832
- Maciejewska-Markiewicz D, Stachowska E, Hawrylkowicz V, Stachowska L, Prowans P. The Role of Resolvins, Protectins and Marensins in Non-Alcoholic Fatty Liver Disease (NAFLD). Biomolecules. Jun 24 2021;11(7)doi:10.3390/biom11070937. https://www.ncbi.nlm.nih.gov/pubmed/34202667
- Duan J, Song Y, Zhang X, Wang C. Effect of omega-3 Polyunsaturated Fatty Acids-Derived Bioactive Lipids on Metabolic Disorders. Front Physiol. 2021;12:646491. doi:10.3389/fphys.2021.646491. https://www.ncbi.nlm.nih.gov/pubmed/34113260
- Green CJ, Pramfalk C, Charlton CA, et al. Hepatic de novo lipogenesis is suppressed and fat oxidation is increased by omega-3 fatty acids at the expense of glucose metabolism. BMJ Open Diabetes Res Care. Mar 2020;8(1)doi:10.1136/bmjdrc-2019-000871. https://www.ncbi.nlm.nih.gov/pubmed/32188593
- Sangouni AA, Orang Z, Mozaffari-Khosravi H. Effect of omega-3 supplementation on fatty liver and visceral adiposity indices in diabetic patients with non-alcoholic fatty liver disease: A randomized controlled trial. Clinical nutrition ESPEN. Aug 2021;44:130-135. doi:10.1016/j.clnesp.2021.06.015. https://www.ncbi.nlm.nih.gov/pubmed/34330456
- Sangouni AA, Orang Z, Mozaffari-Khosravi H. Effect of omega-3 supplementation on cardiometabolic indices in diabetic patients with non-alcoholic fatty liver disease: a randomized controlled trial. BMC Nutr. Dec 15 2021;7(1):86. doi:10.1186/s40795-021-00490-8. https://www.ncbi.nlm.nih.gov/pubmed/34911587
- Cansancao K, Citelli M, Carvalho Leite N, et al. Impact of Long-Term Supplementation with Fish Oil in Individuals with Non-Alcoholic Fatty Liver Disease: A Double Blind Randomized Placebo Controlled Clinical Trial. Nutrients. Nov 2 2020;12(11)doi:10.3390/nu12113372. https://www.ncbi.nlm.nih.gov/pubmed/33147705
- Shojasaadat F, Ayremlou P, Hashemi A, Mehdizadeh A, Zarrin R. A randomized controlled trial comparing effects of a low-energy diet with n-3 polyunsaturated fatty acid supplementation in patients with non-alcoholic fatty liver disease. Journal of research in medical sciences : the official journal of Isfahan University of Medical Sciences . 2019;24:21. doi:10.4103/jrms.JRMS_282_18. https://www.ncbi.nlm.nih.gov/pubmed/31007691
- Yan JH, Guan BJ, Gao HY, Peng XE. Omega-3 polyunsaturated fatty acid supplementation and non-alcoholic fatty liver disease: A meta-analysis of randomized controlled trials. Medicine. Sep 2018;97(37):e12271. doi:10.1097/MD.0000000000012271. https://www.ncbi.nlm.nih.gov/pubmed/30212963
- Musa-Veloso K, Venditti C, Lee HY, et al. Systematic review and meta-analysis of controlled intervention studies on the effectiveness of long-chain omega-3 fatty acids in patients with nonalcoholic fatty liver disease. Nutrition reviews. Aug 1 2018;76(8):581-602. doi:10.1093/nutrit/nuy022. https://www.ncbi.nlm.nih.gov/pubmed/29917092
- Lee CH, Fu Y, Yang SJ, Chi CC. Effects of Omega-3 Polyunsaturated Fatty Acid Supplementation on Non-Alcoholic Fatty Liver: A Systematic Review and Meta-Analysis. Nutrients. Sep 11 2020;12(9)doi:10.3390/nu12092769. https://www.ncbi.nlm.nih.gov/pubmed/32932796
- Mitrovic M, Sistilli G, Horakova O, Rossmeisl M. Omega-3 phospholipids and obesity-associated NAFLD: Potential mechanisms and therapeutic perspectives. European journal of clinical investigation. Mar 2022;52(3):e13650. doi:10.1111/eci.13650. https://www.ncbi.nlm.nih.gov/pubmed/34291454
- Hodges JK, Sasaki GY, Bruno RS. Anti-inflammatory activities of green tea catechins along the gut-liver axis in nonalcoholic fatty liver disease: lessons learned from preclinical and human studies. J Nutr Biochem . Nov 2020;85:108478. doi:10.1016/j.jnutbio.2020.108478.
- Tang G, Xu Y, Zhang C, Wang N, Li H, Feng Y. Green Tea and Epigallocatechin Gallate (EGCG) for the Management of Nonalcoholic Fatty Liver Diseases (NAFLD): Insights into the Role of Oxidative Stress and Antioxidant Mechanism. Antioxidants (Basel, Switzerland). Jul 5 2021;10(7)doi:10.3390/antiox10071076. https://www.ncbi.nlm.nih.gov/pubmed/34356308
- Hussain M, Habib Ur R, Akhtar L. Therapeutic benefits of green tea extract on various parameters in non-alcoholic fatty liver disease patients. Pak J Med Sci. Jul-Aug 2017;33(4):931-936. doi:10.12669/pjms.334.12571. https://www.ncbi.nlm.nih.gov/pubmed/29067068
- Pezeshki A, Safi S, Feizi A, Askari G, Karami F. The Effect of Green Tea Extract Supplementation on Liver Enzymes in Patients with Nonalcoholic Fatty Liver Disease. International journal of preventive medicine. 2016;7:28. doi:10.4103/2008-7802.173051. https://www.ncbi.nlm.nih.gov/pubmed/26955458
- Sakata R, Nakamura T, Torimura T, Ueno T, Sata M. Green tea with high-density catechins improves liver function and fat infiltration in non-alcoholic fatty liver disease (NAFLD) patients: a double-blind placebo-controlled study. Int J Mol Med. Nov 2013;32(5):989-94. doi:10.3892/ijmm.2013.1503. https://www.ncbi.nlm.nih.gov/pubmed/24065295
- Mansour-Ghanaei F, Hadi A, Pourmasoumi M, Joukar F, Golpour S, Najafgholizadeh A. Green tea as a safe alternative approach for nonalcoholic fatty liver treatment: A systematic review and meta-analysis of clinical trials. Phytother Res. Oct 2018;32(10):1876-1884. doi:10.1002/ptr.6130. https://www.ncbi.nlm.nih.gov/pubmed/29947156
- Akbari M, Ostadmohammadi V, Tabrizi R, et al. The effects of alpha-lipoic acid supplementation on inflammatory markers among patients with metabolic syndrome and related disorders: a systematic review and meta-analysis of randomized controlled trials. Nutr Metab (Lond). 2018;15:39. doi:10.1186/s12986-018-0274-y. https://www.ncbi.nlm.nih.gov/pubmed/29930690
- Jeffrey S, Samraj PI, Raj BS. The Role of Alpha-lipoic Acid Supplementation in the Prevention of Diabetes Complications: A Comprehensive Review of Clinical Trials. Curr Diabetes Rev. 2021;17(9):e011821190404. doi:10.2174/1573399817666210118145550. https://www.ncbi.nlm.nih.gov/pubmed/33461470
- Rahmanabadi A, Mahboob S, Amirkhizi F, Hosseinpour-Arjmand S, Ebrahimi-Mameghani M. Oral alpha-lipoic acid supplementation in patients with non-alcoholic fatty liver disease: effects on adipokines and liver histology features. Food Funct. Aug 1 2019;10(8):4941-4952. doi:10.1039/c9fo00449a. https://www.ncbi.nlm.nih.gov/pubmed/31343010
- Hosseinpour-Arjmand S, Amirkhizi F, Ebrahimi-Mameghani M. The effect of alpha-lipoic acid on inflammatory markers and body composition in obese patients with non-alcoholic fatty liver disease: A randomized, double-blind, placebo-controlled trial. J Clin Pharm Ther. Apr 2019;44(2):258-267. doi:10.1111/jcpt.12784. https://www.ncbi.nlm.nih.gov/pubmed/30585337
- Gianturco V, Troisi G, Bellomo A, et al. Impact of combined therapy with alpha-lipoic and ursodeoxycolic acid on nonalcoholic fatty liver disease: double-blind, randomized clinical trial of efficacy and safety. Hepatol Int. Jun 2013;7(2):570-6. doi:10.1007/s12072-012-9387-y. https://www.ncbi.nlm.nih.gov/pubmed/26201789
- Dludla PV, Nkambule BB, Mazibuko-Mbeje SE, et al. N-Acetyl Cysteine Targets Hepatic Lipid Accumulation to Curb Oxidative Stress and Inflammation in NAFLD: A Comprehensive Analysis of the Literature. Antioxidants (Basel, Switzerland). Dec 16 2020;9(12)doi:10.3390/antiox9121283. https://www.ncbi.nlm.nih.gov/pubmed/33339155
- Khoshbaten M, Aliasgarzadeh A, Masnadi K, et al. N-acetylcysteine improves liver function in patients with non-alcoholic Fatty liver disease. Hepatitis monthly. Winter 2010;10(1):12-6. https://www.ncbi.nlm.nih.gov/pubmed/22308119
- Oliveira CP, Cotrim HP, Stefano JT, Siqueira ACG, Salgado ALA, Parise ER. N-Acetylcysteine and/or Ursodeoxycholic Acid Associated with Metformin in Non-Alcoholic Steatohepatitis: An Open-Label Multicenter Randomized Controlled Trial. Arq Gastroenterol. Aug 13 2019;56(2):184-190. doi:10.1590/S0004-2803.201900000-36. https://www.ncbi.nlm.nih.gov/pubmed/31460584
- Pamuk GE, Sonsuz A. N-acetylcysteine in the treatment of non-alcoholic steatohepatitis. Journal of gastroenterology and hepatology. Oct 2003;18(10):1220-1. doi:10.1046/j.1440-1746.2003.03156.x. https://www.ncbi.nlm.nih.gov/pubmed/12974918
- Gutierrez-Mariscal FM, Arenas-de Larriva AP, Limia-Perez L, Romero-Cabrera JL, Yubero-Serrano EM, Lopez-Miranda J. Coenzyme Q10 Supplementation for the Reduction of Oxidative Stress: Clinical Implications in the Treatment of Chronic Diseases. International journal of molecular sciences. Oct 23 2020;21(21)doi:10.3390/ijms21217870. https://www.ncbi.nlm.nih.gov/pubmed/33114148
- Dludla PV, Orlando P, Silvestri S, et al. Coenzyme Q10 Supplementation Improves Adipokine Levels and Alleviates Inflammation and Lipid Peroxidation in Conditions of Metabolic Syndrome: A Meta-Analysis of Randomized Controlled Trials. International journal of molecular sciences. May 4 2020;21(9)doi:10.3390/ijms21093247. https://www.ncbi.nlm.nih.gov/pubmed/32375340
- Ajith TA. Role of mitochondria and mitochondria-targeted agents in non-alcoholic fatty liver disease. Clin Exp Pharmacol Physiol. May 2018;45(5):413-421. doi:10.1111/1440-1681.12886. https://www.ncbi.nlm.nih.gov/pubmed/29112771
- Peng KY, Watt MJ, Rensen S, et al. Mitochondrial dysfunction-related lipid changes occur in nonalcoholic fatty liver disease progression. Journal of lipid research. Oct 2018;59(10):1977-1986. doi:10.1194/jlr.M085613. https://www.ncbi.nlm.nih.gov/pubmed/30042157
- Botham KM, Napolitano M, Bravo E. The Emerging Role of Disturbed CoQ Metabolism in Nonalcoholic Fatty Liver Disease Development and Progression. Nutrients. Dec 1 2015;7(12):9834-46. doi:10.3390/nu7125501. https://www.ncbi.nlm.nih.gov/pubmed/26633474
- Farhangi MA, Alipour B, Jafarvand E, Khoshbaten M. Oral coenzyme Q10 supplementation in patients with nonalcoholic fatty liver disease: effects on serum vaspin, chemerin, pentraxin 3, insulin resistance and oxidative stress. Arch Med Res. Oct 2014;45(7):589-95. doi:10.1016/j.arcmed.2014.11.001. https://www.ncbi.nlm.nih.gov/pubmed/25450583
- Farsi F, Mohammadshahi M, Alavinejad P, Rezazadeh A, Zarei M, Engali KA. Functions of Coenzyme Q10 Supplementation on Liver Enzymes, Markers of Systemic Inflammation, and Adipokines in Patients Affected by Nonalcoholic Fatty Liver Disease: A Double-Blind, Placebo-Controlled, Randomized Clinical Trial. J Am Coll Nutr. May-Jun 2016;35(4):346-53. doi:10.1080/07315724.2015.1021057. https://www.ncbi.nlm.nih.gov/pubmed/26156412
- Melguizo-Rodriguez L, Garcia-Recio E, Ruiz C, De Luna-Bertos E, Illescas-Montes R, Costela-Ruiz VJ. Biological properties and therapeutic applications of garlic and its components. Food Funct. Mar 7 2022;13(5):2415-2426. doi:10.1039/d1fo03180e. https://www.ncbi.nlm.nih.gov/pubmed/35174827
- Soleimani D, Parisa Moosavian S, Zolfaghari H, Paknahad Z. Effect of garlic powder supplementation on blood pressure and hs-C-reactive protein among nonalcoholic fatty liver disease patients: A randomized, double-blind, placebo-controlled trial. Food science & nutrition. Jul 2021;9(7):3556-3562. doi:10.1002/fsn3.2307. https://www.ncbi.nlm.nih.gov/pubmed/34262716
- Soleimani D, Paknahad Z, Rouhani MH. Therapeutic Effects of Garlic on Hepatic Steatosis in Nonalcoholic Fatty Liver Disease Patients: A Randomized Clinical Trial. Diabetes, metabolic syndrome and obesity : targets and therapy. 2020;13:2389-2397. doi:10.2147/DMSO.S254555. https://www.ncbi.nlm.nih.gov/pubmed/32753923
- Soleimani D, Paknahad Z, Askari G, Iraj B, Feizi A. Effect of garlic powder consumption on body composition in patients with nonalcoholic fatty liver disease: A randomized, double-blind, placebo-controlled trial. Advanced biomedical research. 2016;5:2. doi:10.4103/2277-9175.174962. https://www.ncbi.nlm.nih.gov/pubmed/26955623
- Sangouni AA, Alizadeh M, Jamalzehi A, Parastouei K. Effects of garlic powder supplementation on metabolic syndrome components, insulin resistance, fatty liver index, and appetite in subjects with metabolic syndrome: A randomized clinical trial. Phytother Res. Aug 2021;35(8):4433-4441. doi:10.1002/ptr.7146. https://www.ncbi.nlm.nih.gov/pubmed/33974725
- Sangouni AA, Mohammad Hosseini Azar MR, Alizadeh M. Effect of garlic powder supplementation on hepatic steatosis, liver enzymes and lipid profile in patients with non-alcoholic fatty liver disease: a double-blind randomised controlled clinical trial. The British journal of nutrition. Aug 28 2020;124(4):450-456. doi:10.1017/S0007114520001403. https://www.ncbi.nlm.nih.gov/pubmed/32312333
- Sangouni AA, Mohammad Hosseini Azar MR, Alizadeh M. Effects of garlic powder supplementation on insulin resistance, oxidative stress, and body composition in patients with non-alcoholic fatty liver disease: A randomized controlled clinical trial. Complementary therapies in medicine. Jun 2020;51:102428. doi:10.1016/j.ctim.2020.102428. https://www.ncbi.nlm.nih.gov/pubmed/32507439
- NIH. National Institutes of Health: Office of Dietary Supplements. Choline. Last updated 03/29/2021. Accessed 02/21/2022. 2021;https://ods.od.nih.gov/factsheets/Choline-HealthProfessional/
- Sherriff JL, O'Sullivan TA, Properzi C, Oddo JL, Adams LA. Choline, Its Potential Role in Nonalcoholic Fatty Liver Disease, and the Case for Human and Bacterial Genes. Adv Nutr. Jan 2016;7(1):5-13. doi:10.3945/an.114.007955. https://www.ncbi.nlm.nih.gov/pubmed/26773011
- Maev IV, Samsonov AA, Palgova LK, et al. Effectiveness of phosphatidylcholine as adjunctive therapy in improving liver function tests in patients with non-alcoholic fatty liver disease and metabolic comorbidities: real-life observational study from Russia. BMJ open gastroenterology. 2020;7(1):e000368. doi:10.1136/bmjgast-2019-000368. https://www.ncbi.nlm.nih.gov/pubmed/32337059
- Maev IV, Samsonov AA, Palgova LK, et al. Effectiveness of phosphatidylcholine in alleviating steatosis in patients with non-alcoholic fatty liver disease and cardiometabolic comorbidities (MANPOWER study). BMJ open gastroenterology. 2020;7(1):e000341. doi:10.1136/bmjgast-2019-000341. https://www.ncbi.nlm.nih.gov/pubmed/32095253
- Petyaev IM, Dovgalevsky PY, Chalyk NE, Klochkov VA, Kyle NH, Bashmakov YK. Reduction of Liver Span and Parameters of Inflammation in Nonalcoholic Fatty Liver Disease Patients Treated with Lycosome Formulation of Phosphatidylcholine: A Preliminary Report. Int J Chronic Dis. 2018;2018:4549614. doi:10.1155/2018/4549614. https://www.ncbi.nlm.nih.gov/pubmed/29805971
- Elvira-Torales LI, Garcia-Alonso J, Periago-Caston MJ. Nutritional Importance of Carotenoids and Their Effect on Liver Health: A Review. Antioxidants (Basel, Switzerland). Jul 19 2019;8(7)doi:10.3390/antiox8070229. https://www.ncbi.nlm.nih.gov/pubmed/31330977
- Iqbal WA, Mendes I, Finney K, Oxley A, Lietz G. Reduced plasma carotenoids in individuals suffering from metabolic diseases with disturbances in lipid metabolism: a systematic review and meta-analysis of observational studies. International journal of food sciences and nutrition. Nov 2021;72(7):879-891. doi:10.1080/09637486.2021.1882962. https://www.ncbi.nlm.nih.gov/pubmed/33586569
- Clugston RD. Carotenoids and fatty liver disease: Current knowledge and research gaps. Biochimica et biophysica acta Molecular and cell biology of lipids . Nov 2020;1865(11):158597. doi:10.1016/j.bbalip.2019.158597. https://www.ncbi.nlm.nih.gov/pubmed/31904420
- Christensen K, Lawler T, Mares J. Dietary Carotenoids and Non-Alcoholic Fatty Liver Disease among US Adults, NHANES 2003(-)2014. Nutrients . May 17 2019;11(5)doi:10.3390/nu11051101. https://www.ncbi.nlm.nih.gov/pubmed/31108934
- Cao Y, Wang C, Liu J, Liu ZM, Ling WH, Chen YM. Greater serum carotenoid levels associated with lower prevalence of nonalcoholic fatty liver disease in Chinese adults. Sci Rep. Aug 10 2015;5:12951. doi:10.1038/srep12951. https://www.ncbi.nlm.nih.gov/pubmed/26256414
- Xiao ML, Chen GD, Zeng FF, et al. Higher serum carotenoids associated with improvement of non-alcoholic fatty liver disease in adults: a prospective study. European journal of nutrition. Mar 2019;58(2):721-730. doi:10.1007/s00394-018-1678-1. https://www.ncbi.nlm.nih.gov/pubmed/29594435
- Lee Y, Hu S, Park YK, Lee JY. Health Benefits of Carotenoids: A Role of Carotenoids in the Prevention of Non-Alcoholic Fatty Liver Disease. Prev Nutr Food Sci. Jun 2019;24(2):103-113. doi:10.3746/pnf.2019.24.2.103. https://www.ncbi.nlm.nih.gov/pubmed/31328113
- Matsuura B, Miyake T, Yamamoto S, Furukawa S, Hiasa Y. Usefulness of Beta-Cryptoxanthin for Nonalcoholic Fatty Liver. J Food Nutr Disor . 2016;5(3)
- Sahebkar A. Potential efficacy of ginger as a natural supplement for nonalcoholic fatty liver disease. World J Gastroenterol. Jan 14 2011;17(2):271-2. doi:10.3748/wjg.v17.i2.271. https://www.ncbi.nlm.nih.gov/pubmed/21246004
- Rahimlou M, Yari Z, Hekmatdoost A, Alavian SM, Keshavarz SA. Ginger Supplementation in Nonalcoholic Fatty Liver Disease: A Randomized, Double-Blind, Placebo-Controlled Pilot Study. Hepatitis monthly. Jan 2016;16(1):e34897. doi:10.5812/hepatmon.34897. https://www.ncbi.nlm.nih.gov/pubmed/27110262
- Rafie R, Hosseini SA, Hajiani E, Saki Malehi A, Mard SA. Effect of Ginger Powder Supplementation in Patients with Non-Alcoholic Fatty Liver Disease: A Randomized Clinical Trial. Clin Exp Gastroenterol. 2020;13:35-45. doi:10.2147/CEG.S234698. https://www.ncbi.nlm.nih.gov/pubmed/32158249
- Vancells Lujan P, Vinas Esmel E, Sacanella Meseguer E. Overview of Non-Alcoholic Fatty Liver Disease (NAFLD) and the Role of Sugary Food Consumption and Other Dietary Components in Its Development. Nutrients. Apr 24 2021;13(5)doi:10.3390/nu13051442. https://www.ncbi.nlm.nih.gov/pubmed/33923255
- Aboubakr A, Stroud A, Kumar S, Newberry C. Dietary Approaches for Management of Non-Alcoholic Fatty Liver Disease: A Clinician's Guide. Current gastroenterology reports. Oct 15 2021;23(12):21. doi:10.1007/s11894-021-00827-0. https://www.ncbi.nlm.nih.gov/pubmed/34654976
- Armandi A, Schattenberg JM. Beyond the Paradigm of Weight Loss in Non-Alcoholic Fatty Liver Disease: From Pathophysiology to Novel Dietary Approaches. Nutrients. Jun 8 2021;13(6)doi:10.3390/nu13061977. https://www.ncbi.nlm.nih.gov/pubmed/34201382
- Patikorn C, Roubal K, Veettil SK, et al. Intermittent Fasting and Obesity-Related Health Outcomes: An Umbrella Review of Meta-analyses of Randomized Clinical Trials. JAMA Netw Open. Dec 1 2021;4(12):e2139558. doi:10.1001/jamanetworkopen.2021.39558. https://www.ncbi.nlm.nih.gov/pubmed/34919135
- Hassani Zadeh S, Mansoori A, Hosseinzadeh M. Relationship between dietary patterns and non-alcoholic fatty liver disease: A systematic review and meta-analysis. Journal of gastroenterology and hepatology. Jun 2021;36(6):1470-1478. doi:10.1111/jgh.15363. https://www.ncbi.nlm.nih.gov/pubmed/33269500
- Xiao ML, Lin JS, Li YH, et al. Adherence to the Dietary Approaches to Stop Hypertension (DASH) diet is associated with lower presence of non-alcoholic fatty liver disease in middle-aged and elderly adults. Public health nutrition. Mar 2020;23(4):674-682. doi:10.1017/S1368980019002568. https://www.ncbi.nlm.nih.gov/pubmed/31566148
- Alferink LJM, Erler NS, de Knegt RJ, et al. Adherence to a plant-based, high-fibre dietary pattern is related to regression of non-alcoholic fatty liver disease in an elderly population. European journal of epidemiology. Nov 2020;35(11):1069-1085. doi:10.1007/s10654-020-00627-2. https://www.ncbi.nlm.nih.gov/pubmed/32323115
- Mazidi M, Kengne AP. Higher adherence to plant-based diets are associated with lower likelihood of fatty liver. Clin Nutr. Aug 2019;38(4):1672-1677. doi:10.1016/j.clnu.2018.08.010. https://www.ncbi.nlm.nih.gov/pubmed/30578029
- He K, Li Y, Guo X, Zhong L, Tang S. Food groups and the likelihood of non-alcoholic fatty liver disease: a systematic review and meta-analysis. The British journal of nutrition. Mar 6 2020;124(1):1-13. doi:10.1017/S0007114520000914. https://www.ncbi.nlm.nih.gov/pubmed/32138796
- Bahrami A, Teymoori F, Eslamparast T, et al. Legume intake and risk of nonalcoholic fatty liver disease. Indian journal of gastroenterology : official journal of the Indian Society of Gastroenterology . Feb 2019;38(1):55-60. doi:10.1007/s12664-019-00937-8. https://www.ncbi.nlm.nih.gov/pubmed/30796701
- Tarantino G, Citro V, Finelli C. Hype or Reality: Should Patients with Metabolic Syndrome-related NAFLD be on the Hunter-Gatherer (Paleo) Diet to Decrease Morbidity? Journal of gastrointestinal and liver diseases : JGLD. Sep 2015;24(3):359-68. doi:10.15403/jgld.2014.1121.243.gta. https://www.ncbi.nlm.nih.gov/pubmed/26405708
- Moore MP, Cunningham RP, Dashek RJ, Mucinski JM, Rector RS. A Fad too Far? Dietary Strategies for the Prevention and Treatment of NAFLD. Obesity (Silver Spring). Oct 2020;28(10):1843-1852. doi:10.1002/oby.22964. https://www.ncbi.nlm.nih.gov/pubmed/32893456
- Yaskolka Meir A, Rinott E, Tsaban G, et al. Effect of green-Mediterranean diet on intrahepatic fat: the DIRECT PLUS randomised controlled trial. Gut. Nov 2021;70(11):2085-2095. doi:10.1136/gutjnl-2020-323106. https://www.ncbi.nlm.nih.gov/pubmed/33461965
- Roeb E, Weiskirchen R. Fructose and Non-Alcoholic Steatohepatitis. Frontiers in pharmacology. 2021;12:634344. doi:10.3389/fphar.2021.634344. https://www.ncbi.nlm.nih.gov/pubmed/33628193
- Federico A, Rosato V, Masarone M, et al. The Role of Fructose in Non-Alcoholic Steatohepatitis: Old Relationship and New Insights. Nutrients. Apr 16 2021;13(4)doi:10.3390/nu13041314. https://www.ncbi.nlm.nih.gov/pubmed/33923525
- Sevastianova K, Santos A, Kotronen A, et al. Effect of short-term carbohydrate overfeeding and long-term weight loss on liver fat in overweight humans. Am J Clin Nutr. Oct 2012;96(4):727-34. doi:10.3945/ajcn.112.038695. https://www.ncbi.nlm.nih.gov/pubmed/22952180
- Simons N, Veeraiah P, Simons P, et al. Effects of fructose restriction on liver steatosis (FRUITLESS); a double-blind randomized controlled trial. Am J Clin Nutr. Feb 2 2021;113(2):391-400. doi:10.1093/ajcn/nqaa332. https://www.ncbi.nlm.nih.gov/pubmed/33381794
- Schwimmer JB, Ugalde-Nicalo P, Welsh JA, et al. Effect of a Low Free Sugar Diet vs Usual Diet on Nonalcoholic Fatty Liver Disease in Adolescent Boys: A Randomized Clinical Trial. JAMA. Jan 22 2019;321(3):256-265. doi:10.1001/jama.2018.20579. https://www.ncbi.nlm.nih.gov/pubmed/30667502
- Jin R, Welsh JA, Le NA, et al. Dietary fructose reduction improves markers of cardiovascular disease risk in Hispanic-American adolescents with NAFLD. Nutrients. Aug 8 2014;6(8):3187-201. doi:10.3390/nu6083187. https://www.ncbi.nlm.nih.gov/pubmed/25111123
- Dominguez-Coello S, Carrillo-Fernandez L, Gobierno-Hernandez J, et al. Decreased Consumption of Added Fructose Reduces Waist Circumference and Blood Glucose Concentration in Patients with Overweight and Obesity. The DISFRUTE Study: A Randomised Trial in Primary Care. Nutrients. Apr 19 2020;12(4)doi:10.3390/nu12041149. https://www.ncbi.nlm.nih.gov/pubmed/32325919
- Eng JM, Estall JL. Diet-Induced Models of Non-Alcoholic Fatty Liver Disease: Food for Thought on Sugar, Fat, and Cholesterol. Cells. Jul 16 2021;10(7)doi:10.3390/cells10071805. https://www.ncbi.nlm.nih.gov/pubmed/34359974
- Im YR, Hunter H, de Gracia Hahn D, et al. A Systematic Review of Animal Models of NAFLD Finds High-Fat, High-Fructose Diets Most Closely Resemble Human NAFLD. Hepatology (Baltimore, Md). Oct 2021;74(4):1884-1901. doi:10.1002/hep.31897. https://www.ncbi.nlm.nih.gov/pubmed/33973269
- Velazquez AM, Bentanachs R, Sala-Vila A, et al. ChREBP-driven DNL and PNPLA3 Expression Induced by Liquid Fructose are Essential in the Production of Fatty Liver and Hypertriglyceridemia in a High-Fat Diet-Fed Rat Model. Mol Nutr Food Res. Apr 2022;66(7):e2101115. doi:10.1002/mnfr.202101115. https://www.ncbi.nlm.nih.gov/pubmed/35124887
- Mai BH, Yan LJ. The negative and detrimental effects of high fructose on the liver, with special reference to metabolic disorders. Diabetes, metabolic syndrome and obesity : targets and therapy. 2019;12:821-826. doi:10.2147/DMSO.S198968. https://www.ncbi.nlm.nih.gov/pubmed/31213868
- Skenderian S, Park G, Jang C. Organismal Fructose Metabolism in Health and Non-Alcoholic Fatty Liver Disease. Biology. Nov 18 2020;9(11)doi:10.3390/biology9110405. https://www.ncbi.nlm.nih.gov/pubmed/33218081
- Yu S, Li C, Ji G, Zhang L. The Contribution of Dietary Fructose to Non-alcoholic Fatty Liver Disease. Frontiers in pharmacology. 2021;12:783393. doi:10.3389/fphar.2021.783393. https://www.ncbi.nlm.nih.gov/pubmed/34867414
- Asgari-Taee F, Zerafati-Shoae N, Dehghani M, Sadeghi M, Baradaran HR, Jazayeri S. Association of sugar sweetened beverages consumption with non-alcoholic fatty liver disease: a systematic review and meta-analysis. European journal of nutrition. Aug 2019;58(5):1759-1769. doi:10.1007/s00394-018-1711-4. https://www.ncbi.nlm.nih.gov/pubmed/29761318
- Chen H, Wang J, Li Z, et al. Consumption of Sugar-Sweetened Beverages Has a Dose-Dependent Effect on the Risk of Non-Alcoholic Fatty Liver Disease: An Updated Systematic Review and Dose-Response Meta-Analysis. International journal of environmental research and public health. Jun 21 2019;16(12)doi:10.3390/ijerph16122192. https://www.ncbi.nlm.nih.gov/pubmed/31234281
- Li HY, Gan RY, Shang A, et al. Plant-Based Foods and Their Bioactive Compounds on Fatty Liver Disease: Effects, Mechanisms, and Clinical Application. Oxid Med Cell Longev. 2021;2021:6621644. doi:10.1155/2021/6621644. https://www.ncbi.nlm.nih.gov/pubmed/33728021
- Jovanovic-Malinovska R, Kuzmanova S, Winkelhausen E. Oligosaccharide Profile in Fruits and Vegetables as Sources of Prebiotics and Functional Foods. International Journal of Food Properties. 2014/05/28 2014;17(5):949-965. doi:10.1080/10942912.2012.680221. https://doi.org/10.1080/10942912.2012.680221
- Walker RW, Dumke KA, Goran MI. Fructose content in popular beverages made with and without high-fructose corn syrup. Nutrition (Burbank, Los Angeles County, Calif). Jul-Aug 2014;30(7-8):928-35. doi:10.1016/j.nut.2014.04.003. https://www.ncbi.nlm.nih.gov/pubmed/24985013
- Riazi K, Raman M, Taylor L, Swain MG, Shaheen AA. Dietary Patterns and Components in Nonalcoholic Fatty Liver Disease (NAFLD): What Key Messages Can Health Care Providers Offer? Nutrients. Nov 26 2019;11(12)doi:10.3390/nu11122878. https://www.ncbi.nlm.nih.gov/pubmed/31779112
- Zhang S, Wu X, Bian S, et al. Association between consumption frequency of honey and non-alcoholic fatty liver disease: results from a cross-sectional analysis based on the Tianjin Chronic Low-grade Systemic Inflammation and Health (TCLSIH) Cohort Study. The British journal of nutrition. Mar 28 2021;125(6):712-720. doi:10.1017/S0007114520003190. https://www.ncbi.nlm.nih.gov/pubmed/32799936
- Hydes T, Alam U, Cuthbertson DJ. The Impact of Macronutrient Intake on Non-alcoholic Fatty Liver Disease (NAFLD): Too Much Fat, Too Much Carbohydrate, or Just Too Many Calories? Frontiers in nutrition. 2021;8:640557. doi:10.3389/fnut.2021.640557. https://www.ncbi.nlm.nih.gov/pubmed/33665203
- Rosqvist F, Kullberg J, Stahlman M, et al. Overeating Saturated Fat Promotes Fatty Liver and Ceramides Compared With Polyunsaturated Fat: A Randomized Trial. J Clin Endocrinol Metab. Dec 1 2019;104(12):6207-6219. doi:10.1210/jc.2019-00160. https://www.ncbi.nlm.nih.gov/pubmed/31369090
- Luukkonen PK, Sadevirta S, Zhou Y, et al. Saturated Fat Is More Metabolically Harmful for the Human Liver Than Unsaturated Fat or Simple Sugars. Diabetes Care. Aug 2018;41(8):1732-1739. doi:10.2337/dc18-0071. https://www.ncbi.nlm.nih.gov/pubmed/29844096
- Bjermo H, Iggman D, Kullberg J, et al. Effects of n-6 PUFAs compared with SFAs on liver fat, lipoproteins, and inflammation in abdominal obesity: a randomized controlled trial. Am J Clin Nutr. May 2012;95(5):1003-12. doi:10.3945/ajcn.111.030114. https://www.ncbi.nlm.nih.gov/pubmed/22492369
- Markova M, Pivovarova O, Hornemann S, et al. Isocaloric Diets High in Animal or Plant Protein Reduce Liver Fat and Inflammation in Individuals With Type 2 Diabetes. Gastroenterology. Feb 2017;152(3):571-585 e8. doi:10.1053/j.gastro.2016.10.007. https://www.ncbi.nlm.nih.gov/pubmed/27765690
- Hayat U, Siddiqui AA, Okut H, Afroz S, Tasleem S, Haris A. The effect of coffee consumption on the non-alcoholic fatty liver disease and liver fibrosis: A meta-analysis of 11 epidemiological studies. Ann Hepatol. Jan-Feb 2021;20:100254. doi:10.1016/j.aohep.2020.08.071. https://www.ncbi.nlm.nih.gov/pubmed/32920163
- Sewter R, Heaney S, Patterson A. Coffee Consumption and the Progression of NAFLD: A Systematic Review. Nutrients. Jul 12 2021;13(7)doi:10.3390/nu13072381. https://www.ncbi.nlm.nih.gov/pubmed/34371891
- Kositamongkol C, Kanchanasurakit S, Auttamalang C, et al. Coffee Consumption and Non-alcoholic Fatty Liver Disease: An Umbrella Review and a Systematic Review and Meta-analysis. Frontiers in pharmacology. 2021;12:786596. doi:10.3389/fphar.2021.786596. https://www.ncbi.nlm.nih.gov/pubmed/34966282
- Ebadi M, Ip S, Bhanji RA, Montano-Loza AJ. Effect of Coffee Consumption on Non-Alcoholic Fatty Liver Disease Incidence, Prevalence and Risk of Significant Liver Fibrosis: Systematic Review with Meta-Analysis of Observational Studies. Nutrients. Aug 30 2021;13(9)doi:10.3390/nu13093042. https://www.ncbi.nlm.nih.gov/pubmed/34578919
- Nikrandt G, Chmurzynska A. Coffee and nonalcoholic fatty liver disease: A review. Acta scientiarum polonorum Technologia alimentaria. Jul-Sep 2020;19(3):245-254. doi:10.17306/J.AFS.0796. https://www.ncbi.nlm.nih.gov/pubmed/32978907
- Kennedy OJ, Fallowfield JA, Poole R, Hayes PC, Parkes J, Roderick PJ. All coffee types decrease the risk of adverse clinical outcomes in chronic liver disease: a UK Biobank study. BMC Public Health. Jun 22 2021;21(1):970. doi:10.1186/s12889-021-10991-7. https://www.ncbi.nlm.nih.gov/pubmed/34154561
- Sebti Y, Hebras A, Pourcet B, Staels B, Duez H. The Circadian Clock and Obesity. Handbook of experimental pharmacology. Feb 3 2022;doi:10.1007/164_2021_579. https://www.ncbi.nlm.nih.gov/pubmed/35112237
- Nishi T, Babazono A, Maeda T, Imatoh T, Une H. Effects of Eating Fast and Eating Before Bedtime on the Development of Nonalcoholic Fatty Liver Disease. Popul Health Manag. Aug 2016;19(4):279-83. doi:10.1089/pop.2015.0088. https://www.ncbi.nlm.nih.gov/pubmed/26565781
- Gu C, Brereton N, Schweitzer A, et al. Metabolic Effects of Late Dinner in Healthy Volunteers-A Randomized Crossover Clinical Trial. J Clin Endocrinol Metab. Aug 1 2020;105(8):2789-802. doi:10.1210/clinem/dgaa354. https://www.ncbi.nlm.nih.gov/pubmed/32525525
- Lee S, Ko BJ, Gong Y, et al. Self-reported eating speed in relation to non-alcoholic fatty liver disease in adults. European journal of nutrition. Feb 2016;55(1):327-33. doi:10.1007/s00394-015-0851-z. https://www.ncbi.nlm.nih.gov/pubmed/25648740
- Cao X, Gu Y, Bian S, et al. Association between eating speed and newly diagnosed nonalcoholic fatty liver disease among the general population. Nutr Res. Aug 2020;80:78-88. doi:10.1016/j.nutres.2020.06.012. https://www.ncbi.nlm.nih.gov/pubmed/32736293
- Cigrovski Berkovic M, Bilic-Curcic I, Mrzljak A, Cigrovski V. NAFLD and Physical Exercise: Ready, Steady, Go! Frontiers in nutrition. 2021;8:734859. doi:10.3389/fnut.2021.734859. https://www.ncbi.nlm.nih.gov/pubmed/34676233
- Machado MV. Aerobic Exercise in the Management of Metabolic Dysfunction Associated Fatty Liver Disease. Diabetes, metabolic syndrome and obesity : targets and therapy. 2021;14:3627-3645. doi:10.2147/DMSO.S304357. https://www.ncbi.nlm.nih.gov/pubmed/34408459
- Sung KC, Ryu S, Lee JY, Kim JY, Wild SH, Byrne CD. Effect of exercise on the development of new fatty liver and the resolution of existing fatty liver. J Hepatol. Oct 2016;65(4):791-797. doi:10.1016/j.jhep.2016.05.026. https://www.ncbi.nlm.nih.gov/pubmed/27255583
- Gerage AM, Ritti-Dias RM, Balagopal PB, et al. Physical activity levels and hepatic steatosis: A longitudinal follow-up study in adults. Journal of gastroenterology and hepatology. Mar 2018;33(3):741-746. doi:10.1111/jgh.13965. https://www.ncbi.nlm.nih.gov/pubmed/28857324
- Babu AF, Csader S, Lok J, et al. Positive Effects of Exercise Intervention without Weight Loss and Dietary Changes in NAFLD-Related Clinical Parameters: A Systematic Review and Meta-Analysis. Nutrients. Sep 8 2021;13(9)doi:10.3390/nu13093135. https://www.ncbi.nlm.nih.gov/pubmed/34579012
- Kanwal F, Shubrook JH, Adams LA, et al. Clinical Care Pathway for the Risk Stratification and Management of Patients With Nonalcoholic Fatty Liver Disease. Gastroenterology. Nov 2021;161(5):1657-1669. doi:10.1053/j.gastro.2021.07.049. https://www.ncbi.nlm.nih.gov/pubmed/34602251
- Mansour D, Grapes A, Herscovitz M, et al. Embedding assessment of liver fibrosis into routine diabetic review in primary care. JHEP Rep. Aug 2021;3(4):100293. doi:10.1016/j.jhepr.2021.100293. https://www.ncbi.nlm.nih.gov/pubmed/34179738
- Vieira Barbosa J, Lai M. Nonalcoholic Fatty Liver Disease Screening in Type 2 Diabetes Mellitus Patients in the Primary Care Setting. Hepatology communications. Feb 2021;5(2):158-167. doi:10.1002/hep4.1618. https://www.ncbi.nlm.nih.gov/pubmed/33553966
- Dorairaj V, Sulaiman SA, Abu N, Abdul Murad NA. Nonalcoholic Fatty Liver Disease (NAFLD): Pathogenesis and Noninvasive Diagnosis. Biomedicines. Dec 22 2021;10(1)doi:10.3390/biomedicines10010015. https://www.ncbi.nlm.nih.gov/pubmed/35052690
- Kalas MA, Chavez L, Leon M, Taweesedt PT, Surani S. Abnormal liver enzymes: A review for clinicians. World J Hepatol. Nov 27 2021;13(11):1688-1698. doi:10.4254/wjh.v13.i11.1688. https://www.ncbi.nlm.nih.gov/pubmed/34904038
- Zhou JH, Cai JJ, She ZG, Li HL. Noninvasive evaluation of nonalcoholic fatty liver disease: Current evidence and practice. World J Gastroenterol. Mar 21 2019;25(11):1307-1326. doi:10.3748/wjg.v25.i11.1307. https://www.ncbi.nlm.nih.gov/pubmed/30918425
- Segura-Azuara NLA, Varela-Chinchilla CD, Trinidad-Calderon PA. MAFLD/NAFLD Biopsy-Free Scoring Systems for Hepatic Steatosis, NASH, and Fibrosis Diagnosis. Front Med (Lausanne). 2021;8:774079. doi:10.3389/fmed.2021.774079. https://www.ncbi.nlm.nih.gov/pubmed/35096868
- Vilar-Gomez E, Lou Z, Kong N, Vuppalanchi R, Imperiale TF, Chalasani N. Cost Effectiveness of Different Strategies for Detecting Cirrhosis in Patients With Nonalcoholic Fatty Liver Disease Based on United States Health Care System. Clinical gastroenterology and hepatology : the official clinical practice journal of the American Gastroenterological Association . Sep 2020;18(10):2305-2314 e12. doi:10.1016/j.cgh.2020.04.017. https://www.ncbi.nlm.nih.gov/pubmed/32289535
- Hashem A, Khalouf A, Acosta A. Management of Obesity and Nonalcoholic Fatty Liver Disease: A Literature Review. Semin Liver Dis. Nov 2021;41(4):435-447. doi:10.1055/s-0041-1731704. https://www.ncbi.nlm.nih.gov/pubmed/34243193
- Fan S, Shi X, Yao J, Zhong M, Feng P. The efficacy of glucagon-like peptide 1 receptor agonists in patients with non-alcoholic fatty liver disease: a systematic review and meta-analysis of randomized controlled trials. Revista espanola de enfermedades digestivas : organo oficial de la Sociedad Espanola de Patologia Digestiva . Aug 2020;112(8):627-635. doi:10.17235/reed.2020.6392/2019. https://www.ncbi.nlm.nih.gov/pubmed/32496108
- Kalogirou MS, Patoulias D, Haidich AB, Akriviadis E, Sinakos E. Liraglutide in patients with non-alcoholic fatty liver disease: a systematic review and meta-analysis of randomized controlled trials. Clinics and research in hepatology and gastroenterology. May 2021;45(3):101568. doi:10.1016/j.clinre.2020.10.012. https://www.ncbi.nlm.nih.gov/pubmed/33309563
- Newsome PN, Buchholtz K, Cusi K, et al. A Placebo-Controlled Trial of Subcutaneous Semaglutide in Nonalcoholic Steatohepatitis. The New England journal of medicine. Mar 25 2021;384(12):1113-1124. doi:10.1056/NEJMoa2028395. https://www.ncbi.nlm.nih.gov/pubmed/33185364
- Nahra R, Wang T, Gadde KM, et al. Effects of Cotadutide on Metabolic and Hepatic Parameters in Adults With Overweight or Obesity and Type 2 Diabetes: A 54-Week Randomized Phase 2b Study. Diabetes Care. Jun 2021;44(6):1433-1442. doi:10.2337/dc20-2151. https://www.ncbi.nlm.nih.gov/pubmed/34016612
- Lian J, Fu J. Pioglitazone for NAFLD Patients With Prediabetes or Type 2 Diabetes Mellitus: A Meta-Analysis. Frontiers in endocrinology. 2021;12:615409. doi:10.3389/fendo.2021.615409. https://www.ncbi.nlm.nih.gov/pubmed/33995271
- Manka PP, Kaya E, Canbay A, Syn WK. A Review of the Epidemiology, Pathophysiology, and Efficacy of Anti-diabetic Drugs Used in the Treatment of Nonalcoholic Fatty Liver Disease. Dig Dis Sci. Nov 2021;66(11):3676-3688. doi:10.1007/s10620-021-07206-9. https://www.ncbi.nlm.nih.gov/pubmed/34410573
- Kahl S, Gancheva S, Strassburger K, et al. Empagliflozin Effectively Lowers Liver Fat Content in Well-Controlled Type 2 Diabetes: A Randomized, Double-Blind, Phase 4, Placebo-Controlled Trial. Diabetes Care. Feb 2020;43(2):298-305. doi:10.2337/dc19-0641. https://www.ncbi.nlm.nih.gov/pubmed/31540903
- Kuchay MS, Krishan S, Mishra SK, et al. Effect of Empagliflozin on Liver Fat in Patients With Type 2 Diabetes and Nonalcoholic Fatty Liver Disease: A Randomized Controlled Trial (E-LIFT Trial). Diabetes Care. Aug 2018;41(8):1801-1808. doi:10.2337/dc18-0165. https://www.ncbi.nlm.nih.gov/pubmed/29895557
- Sattar N, Fitchett D, Hantel S, George JT, Zinman B. Empagliflozin is associated with improvements in liver enzymes potentially consistent with reductions in liver fat: results from randomised trials including the EMPA-REG OUTCOME(R) trial. Diabetologia. Oct 2018;61(10):2155-2163. doi:10.1007/s00125-018-4702-3. https://www.ncbi.nlm.nih.gov/pubmed/30066148
- Lee JY, Kim YE, Han K, et al. Analysis of Severe Hypoglycemia Among Adults With Type 2 Diabetes and Nonalcoholic Fatty Liver Disease. JAMA Netw Open. Feb 1 2022;5(2):e220262. doi:10.1001/jamanetworkopen.2022.0262. https://www.ncbi.nlm.nih.gov/pubmed/35195697
- Choi SY, Ko SH. Severe hypoglycemia as a preventable risk factor for cardiovascular disease in patients with type 2 diabetes mellitus. The Korean journal of internal medicine. Mar 2021;36(2):263-270. doi:10.3904/kjim.2020.327. https://www.ncbi.nlm.nih.gov/pubmed/32872725
- Winterstein AG, Jeon N, Staley B, Xu D, Henriksen C, Lipori GP. Development and validation of an automated algorithm for identifying patients at high risk for drug-induced hypoglycemia. American journal of health-system pharmacy : AJHP : official journal of the American Society of Health-System Pharmacists . Nov 1 2018;75(21):1714-1728. doi:10.2146/ajhp180071. https://www.ncbi.nlm.nih.gov/pubmed/30279185
- Westall SJ, Narayanan RP, Watmough S, et al. The individualisation of glycaemic targets in response to patient characteristics in type 2 diabetes: a scoping review. Clinical medicine (London, England). Apr 20 2022;doi:10.7861/clinmed.2021-0764. https://www.ncbi.nlm.nih.gov/pubmed/35443970
- Jagtap N, Kalapala R, Katakwar A, et al. Endoscopic sleeve gastroplasty - minimally invasive treatment for non-alcoholic fatty liver disease and obesity. Indian journal of gastroenterology : official journal of the Indian Society of Gastroenterology . Dec 2021;40(6):572-579. doi:10.1007/s12664-021-01202-7. https://www.ncbi.nlm.nih.gov/pubmed/34914039
- Hajifathalian K, Mehta A, Ang B, et al. Improvement in insulin resistance and estimated hepatic steatosis and fibrosis after endoscopic sleeve gastroplasty. Gastrointest Endosc. May 2021;93(5):1110-1118. doi:10.1016/j.gie.2020.08.023. https://www.ncbi.nlm.nih.gov/pubmed/32861753
- Seymour KA, Abdelmalek MF. The role of bariatric surgery in the management of nonalcoholic steatohepatitis. Current opinion in gastroenterology. May 1 2021;37(3):208-215. doi:10.1097/MOG.0000000000000721. https://www.ncbi.nlm.nih.gov/pubmed/33769376
- Lassailly G, Caiazzo R, Ntandja-Wandji LC, et al. Bariatric Surgery Provides Long-term Resolution of Nonalcoholic Steatohepatitis and Regression of Fibrosis. Gastroenterology. Oct 2020;159(4):1290-1301 e5. doi:10.1053/j.gastro.2020.06.006. https://www.ncbi.nlm.nih.gov/pubmed/32553765
- Aminian A, Al-Kurd A, Wilson R, et al. Association of Bariatric Surgery With Major Adverse Liver and Cardiovascular Outcomes in Patients With Biopsy-Proven Nonalcoholic Steatohepatitis. JAMA. Nov 23 2021;326(20):2031-2042. doi:10.1001/jama.2021.19569. https://www.ncbi.nlm.nih.gov/pubmed/34762106
- Lim R, Beekley A, Johnson DC, Davis KA. Early and late complications of bariatric operation. Trauma Surg Acute Care Open. 2018;3(1):e000219. doi:10.1136/tsaco-2018-000219. https://www.ncbi.nlm.nih.gov/pubmed/30402562
- Filipovic B, Lukic S, Mijac D, et al. The New Therapeutic Approaches in the Treatment of Non-Alcoholic Fatty Liver Disease. International journal of molecular sciences. Dec 8 2021;22(24)doi:10.3390/ijms222413219. https://www.ncbi.nlm.nih.gov/pubmed/34948020
- Borem LMA, Neto JFR, Brandi IV, Lelis DF, Santos SHS. The role of the angiotensin II type I receptor blocker telmisartan in the treatment of non-alcoholic fatty liver disease: a brief review. Hypertension research : official journal of the Japanese Society of Hypertension . Jun 2018;41(6):394-405. doi:10.1038/s41440-018-0040-6. https://www.ncbi.nlm.nih.gov/pubmed/29636553
- Alam S, Abrar M, Islam S, et al. Effect of telmisartan and vitamin E on liver histopathology with non-alcoholic steatohepatitis: A randomized, open-label, noninferiority trial. JGH Open. Aug 2020;4(4):663-669. doi:10.1002/jgh3.12315. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7411541/pdf/JGH3-4-663.pdf
- Alam S, Kabir J, Mustafa G, Gupta U, Hasan SK, Alam AK. Effect of telmisartan on histological activity and fibrosis of non-alcoholic steatohepatitis: A 1-year randomized control trial. Saudi J Gastroenterol. Jan-Feb 2016;22(1):69-76. doi:10.4103/1319-3767.173762. https://www.ncbi.nlm.nih.gov/pubmed/26831610
- Devan AR, Nair B, Kumar AR, Nath LR. An insight into the role of telmisartan as PPAR-gamma/alpha dual activator in the management of nonalcoholic fatty liver disease. Biotechnol Appl Biochem. Apr 2022;69(2):461-468. doi:10.1002/bab.2123. https://www.ncbi.nlm.nih.gov/pubmed/33578449
- Zhang W, Tang Y, Huang J, Hu H. Efficacy of ursodeoxycholic acid in nonalcoholic fatty liver disease: An updated meta-analysis of randomized controlled trials. Asia Pac J Clin Nutr. 2020;29(4):696-705. doi:10.6133/apjcn.202012_29(4).0004. https://www.ncbi.nlm.nih.gov/pubmed/33377363
- Younossi ZM, Ratziu V, Loomba R, et al. Obeticholic acid for the treatment of non-alcoholic steatohepatitis: interim analysis from a multicentre, randomised, placebo-controlled phase 3 trial. Lancet. Dec 14 2019;394(10215):2184-2196. doi:10.1016/S0140-6736(19)33041-7. https://www.ncbi.nlm.nih.gov/pubmed/31813633
- Safadi R, Konikoff FM, Mahamid M, et al. The fatty acid-bile acid conjugate Aramchol reduces liver fat content in patients with nonalcoholic fatty liver disease. Clinical gastroenterology and hepatology : the official clinical practice journal of the American Gastroenterological Association . Dec 2014;12(12):2085-91 e1. doi:10.1016/j.cgh.2014.04.038. https://www.ncbi.nlm.nih.gov/pubmed/24815326
- Loomba R, Kayali Z, Noureddin M, et al. GS-0976 Reduces Hepatic Steatosis and Fibrosis Markers in Patients With Nonalcoholic Fatty Liver Disease. Gastroenterology. Nov 2018;155(5):1463-1473 e6. doi:10.1053/j.gastro.2018.07.027. https://www.ncbi.nlm.nih.gov/pubmed/30059671
- Harrison SA, Bashir MR, Guy CD, et al. Resmetirom (MGL-3196) for the treatment of non-alcoholic steatohepatitis: a multicentre, randomised, double-blind, placebo-controlled, phase 2 trial. Lancet. Nov 30 2019;394(10213):2012-2024. doi:10.1016/S0140-6736(19)32517-6. https://www.ncbi.nlm.nih.gov/pubmed/31727409
- Harrison SA, Bashir M, Moussa SE, et al. Effects of Resmetirom on Noninvasive Endpoints in a 36-Week Phase 2 Active Treatment Extension Study in Patients With NASH. Hepatology communications. Apr 2021;5(4):573-588. doi:10.1002/hep4.1657. https://www.ncbi.nlm.nih.gov/pubmed/33860116
- Ratziu V, Sanyal A, Harrison SA, et al. Cenicriviroc Treatment for Adults With Nonalcoholic Steatohepatitis and Fibrosis: Final Analysis of the Phase 2b CENTAUR Study. Hepatology (Baltimore, Md). Sep 2020;72(3):892-905. doi:10.1002/hep.31108. https://www.ncbi.nlm.nih.gov/pubmed/31943293
- Harrison SA, Neff G, Guy CD, et al. Efficacy and Safety of Aldafermin, an Engineered FGF19 Analog, in a Randomized, Double-Blind, Placebo-Controlled Trial of Patients With Nonalcoholic Steatohepatitis. Gastroenterology. Jan 2021;160(1):219-231 e1. doi:10.1053/j.gastro.2020.08.004. https://www.ncbi.nlm.nih.gov/pubmed/32781086
- Zein CO, Yerian LM, Gogate P, et al. Pentoxifylline improves nonalcoholic steatohepatitis: a randomized placebo-controlled trial. Hepatology (Baltimore, Md). Nov 2011;54(5):1610-9. doi:10.1002/hep.24544. https://www.ncbi.nlm.nih.gov/pubmed/21748765
- Van Wagner LB, Koppe SW, Brunt EM, et al. Pentoxifylline for the treatment of non-alcoholic steatohepatitis: a randomized controlled trial. Ann Hepatol. Jul-Sep 2011;10(3):277-86. https://www.ncbi.nlm.nih.gov/pubmed/21677329
- Wierzbicki AS, Pendleton S, McMahon Z, et al. Rimonabant improves cholesterol, insulin resistance and markers of non-alcoholic fatty liver in morbidly obese patients: a retrospective cohort study. International journal of clinical practice. Jun 2011;65(6):713-5. doi:10.1111/j.1742-1241.2011.02683.x. https://www.ncbi.nlm.nih.gov/pubmed/21564446
- Dawson AJ, Kilpatrick ES, Coady AM, et al. Endocannabinoid receptor blockade reduces alanine aminotransferase in polycystic ovary syndrome independent of weight loss. BMC endocrine disorders. Jul 14 2017;17(1):41. doi:10.1186/s12902-017-0194-2. https://www.ncbi.nlm.nih.gov/pubmed/28705172
- Sathyapalan T, Dakroury Y, Ahmed L, et al. Endocannabinoid receptor blockade increases hepatocyte growth factor and reduces insulin levels in obese women with polycystic ovary syndrome. Clin Endocrinol (Oxf). Oct 2016;85(4):671-3. doi:10.1111/cen.13120. https://onlinelibrary.wiley.com/doi/10.1111/cen.13120
- Mazzotti A, Caletti MT, Brodosi L, et al. An internet-based approach for lifestyle changes in patients with NAFLD: Two-year effects on weight loss and surrogate markers. J Hepatol. Nov 2018;69(5):1155-1163. doi:10.1016/j.jhep.2018.07.013. https://www.ncbi.nlm.nih.gov/pubmed/30290973
- Axley P, Kodali S, Kuo YF, et al. Text messaging approach improves weight loss in patients with nonalcoholic fatty liver disease: A randomized study. Liver Int. May 2018;38(5):924-931. doi:10.1111/liv.13622. https://www.ncbi.nlm.nih.gov/pubmed/29117472
- Weichmann F, Avaltroni F, Burki C. Review of Clinical Effects and Presumed Mechanism of Action of the French Oak Wood Extract Robuvit. Journal of medicinal food. Sep 2021;24(9):897-907. doi:10.1089/jmf.2020.0165. https://www.ncbi.nlm.nih.gov/pubmed/33512270
- Um JH, Yun J. Emerging role of mitophagy in human diseases and physiology. BMB reports. Jun 2017;50(6):299-307. doi:10.5483/bmbrep.2017.50.6.056. doi:10.5483/BMBRep.2017.50.6.056. https://pubmed.ncbi.nlm.nih.gov/28366191/
- Natella F, Leoni G, Maldini M, et al. Absorption, metabolism, and effects at transcriptome level of a standardized French oak wood extract, Robuvit, in healthy volunteers: pilot study. J Agric Food Chem. Jan 15 2014;62(2):443-53. doi:10.1021/jf403493a. https://www.ncbi.nlm.nih.gov/pubmed/24354337
- Belcaro G, Cox DM, Cesarone MR, et al. Effects of Robuvit® on the progression of non-alcoholic fatty liver disease. Minerva Gastroenterol (Torino). Dec 2022;68(4):434-441. doi:10.23736/s2724-5985.22.03201-6. https://pubmed.ncbi.nlm.nih.gov/36507829
- Sharifi S, Bagherniya M, Khoram Z, et al. Efficacy of curcumin plus piperine co-supplementation in moderate-to-high hepatic steatosis: A double-blind, randomized, placebo-controlled clinical trial. Phytother Res. Feb 17 2023;doi:10.1002/ptr.7764.
- Panahi Y, Kianpour P, Mohtashami R, Soflaei SS, Sahebkar A. Efficacy of phospholipidated curcumin in nonalcoholic fatty liver disease: a clinical study. Journal of Asian natural products research. Aug 2019;21(8):798-805. doi:10.1080/10286020.2018.1505873.
- Panahi Y, Kianpour P, Mohtashami R, Jafari R, Simental-Mendía LE, Sahebkar A. Efficacy and Safety of Phytosomal Curcumin in Non-Alcoholic Fatty Liver Disease: A Randomized Controlled Trial. Drug research. Apr 2017;67(4):244-251. doi:10.1055/s-0043-100019.
- Rinella ME, Lazarus JV, Ratziu V, et al. A multi-society Delphi consensus statement on new fatty liver disease nomenclature. J Hepatol. Jun 20 2023;doi:10.1016/j.jhep.2023.06.003.
- FDA. U.S. Food & Drug Administration. FDA News Release: FDA Approves First Treatment for Patients with Liver Scarring Due to Fatty Liver Disease. 3/14/2024. Accessed 4/11/2024. https://www.fda.gov/news-events/press-announcements/fda-approves-first-treatment-patients-liver-scarring-due-fatty-liver-disease
- Harrison SA, Bedossa P, Guy CD, et al. A Phase 3, Randomized, Controlled Trial of Resmetirom in NASH with Liver Fibrosis. The New England journal of medicine. Feb 8 2024;390(6):497-509. doi:10.1056/NEJMoa2309000. https://www.ncbi.nlm.nih.gov/pubmed/38324483
- Harrison SA, Taub R, Neff GW, et al. Resmetirom for nonalcoholic fatty liver disease: a randomized, double-blind, placebo-controlled phase 3 trial. Nat Med. 2023;29(11):2919-2928. doi:10.1038/s41591-023-02603-1. https://doi.org/10.1038/s41591-023-02603-1